J Korean Med Sci.
2014 Sep;29(9):1199-1204. 10.3346/jkms.2014.29.9.1199.
Usefulness of Serum Leucine-Rich Alpha-2 Glycoprotein as a Disease Activity Biomarker in Patients with Rheumatoid Arthritis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 2Division of Rheumatology, Department of Internal Medicine, Busan Medical Center, Busan, Korea.
- 3Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Rheumatology, Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. beconst@hanmail.net
- KMID: 1794596
- DOI: http://doi.org/10.3346/jkms.2014.29.9.1199
Abstract
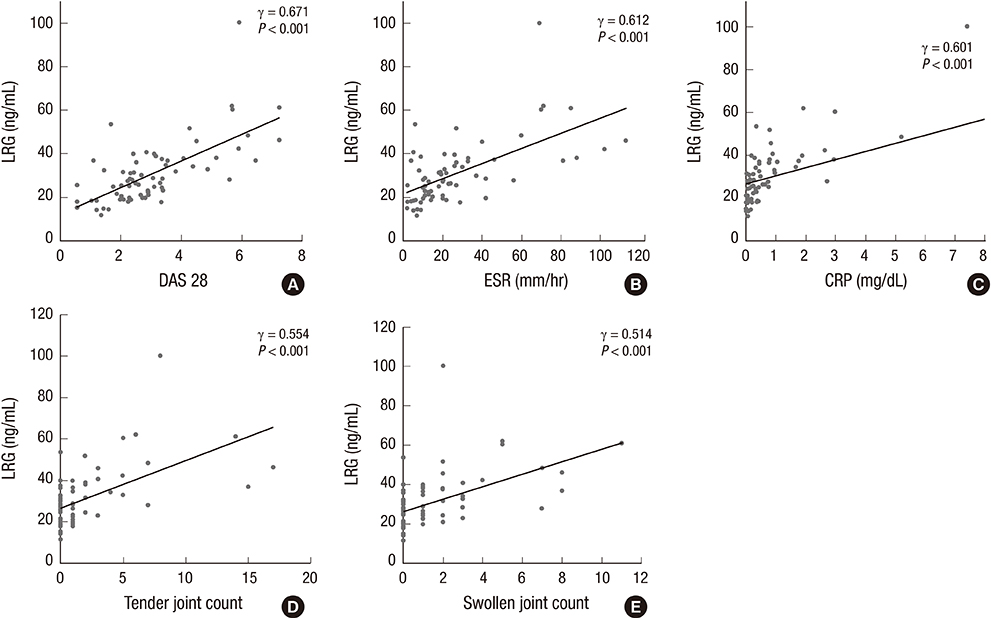
- Our study aimed to investigate whether serum leucine-rich alpha-2-glycoprotein (LRG) levels are elevated in patients with rheumatoid arthritis (RA). In addition, we assessed their correlation with disease activity parameters and pro-inflammatory cytokine, tumor necrosis factor-alpha (TNF-alpha). Our study included 69 patients with RA and 48 age- and sex-matched healthy controls. Serum concentrations of TNF-alpha and LRG were determined by enzyme-linked immunosorbent assay. Serum LRG concentrations were significantly elevated in patients with RA compared with those in healthy controls (30.8+/-14.4 vs. 22.2+/-6.1 ng/mL; P<0.001). In patients with RA, serum LRG levels were found to be correlated with disease activity score 28 (DAS28), erythrocyte sedimentation rate, and C-reactive protein levels (gamma=0.671; gamma=0.612; and gamma=0.601, P<0.001, respectively), but not with serum TNF-alpha levels. Serum LRG levels in patients with an active disease status (DAS28> or =2.6) were significantly higher than those in remission (DAS28<2.6) (36.45+/-14.36 vs. 24.63+/-8.81 ng/mL; P<0.001). Our findings suggest that serum LRG could contribute to the inflammatory process independent of TNF-alpha and it may be a novel biomarker for assessing inflammatory activity in patients with RA.
Keyword
MeSH Terms
-
Adult
Aged
Area Under Curve
Arthritis, Rheumatoid/blood/*diagnosis
Biological Markers/blood
Blood Sedimentation
C-Reactive Protein/analysis
Enzyme-Linked Immunosorbent Assay
Female
Glycoproteins/*blood
Humans
Male
Middle Aged
ROC Curve
*Severity of Illness Index
Tumor Necrosis Factor-alpha/blood
Biological Markers
C-Reactive Protein
Glycoproteins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010; 376:1094–1108.2. Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, Combe B, Cutolo M, de Wit M, Dougados M, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010; 69:631–637.3. Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010; 69:964–975.4. Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One. 2009; 4:e6242.5. Wolfe F. The many myths of erythrocyte sedimentation rate and C-reactive protein. J Rheumatol. 2009; 36:1568–1569.6. Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985; 82:1906–1910.7. Haupt H, Baudner S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author's transl). Hoppe Seylers Z Physiol Chem. 1977; 358:639–646.8. Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, Ohkawara T, Nezu R, Nakajima S, Kobayashi T, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012; 18:2169–2179.9. Nakajima M, Miyajima M, Ogino I, Watanabe M, Hagiwara Y, Segawa T, Kobayashi K, Arai H. Brain localization of leucine-rich α2-glycoprotein and its role. Acta Neurochir Suppl. 2012; 113:97–101.10. Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun. 2009; 382:776–779.11. O'Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002; 72:478–485.12. Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H, Shinzaki S, Nishikawa T, Ohkawara T, Iwahori K, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010; 69:770–774.13. Xie Q, Wang SC, Li J. Interleukin 22, a potential therapeutic target for rheumatoid arthritis. J Rheumatol. 2012; 39:2220.14. Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009; 373:659–672.15. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324.16. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–2581.17. Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995; 38:44–48.18. Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, Oh HJ, Heo YJ, Park SH, Kim HY, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J Korean Med Sci. 2011; 26:1132–1139.19. Altomonte L, Zoli A, Mirone L, Scolieri P, Magaró M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis: correlation with disease activity. Clin Rheumatol. 1992; 11:202–205.20. Yen JH, Chen JR, Tsai WJ, Liu HW. Correlation of tumor necrosis factor alpha levels with disease activity of rheumatoid arthritis. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1992; 25:232–243.21. Wascher TC, Hermann J, Brezinschek R, Brezinschek HP, Wilders-Truschnig M, Rainer F, Krejs GJ. Serum levels of interleukin-6 and tumour-necrosis-factor-alpha are not correlated to disease activity in patients with rheumatoid arthritis after treatment with low-dose methotrexate. Eur J Clin Invest. 1994; 24:73–75.22. Cummings C, Walder J, Treeful A, Jemmerson R. Serum leucine-rich alpha-2-glycoprotein-1 binds cytochrome c and inhibits antibody detection of this apoptotic marker in enzyme-linked immunosorbent assay. Apoptosis. 2006; 11:1121–1129.23. Wang X, Abraham S, McKenzie JA, Jeffs N, Swire M, Tripathi VB, Luhmann UF, Lange CA, Zhai Z, Arthur HM, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013; 499:306–311.24. Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011; 10:595–598.25. Liu H, Pope RM. The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol. 2003; 3:317–322.26. Kentsis A, Lin YY, Kurek K, Calicchio M, Wang YY, Monigatti F, Campagne F, Lee R, Horwitz B, Steen H, et al. Discovery and validation of urine markers of acute pediatric appendicitis using high-accuracy mass spectrometry. Ann Emerg Med. 2010; 55:62–70.e4.27. Choi JW, Liu H, Shin DH, Yu GI, Hwang JS, Kim ES, Yun JW. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients. Proteomics. 2013; 13:2361–2374.28. Liu Y, Luo X, Hu H, Wang R, Sun Y, Zeng R, Chen H. Integrative proteomics and tissue microarray profiling indicate the association between overexpressed serum proteins and non-small cell lung cancer. PLoS One. 2012; 7:e51748.29. Sandanayake NS, Sinclair J, Andreola F, Chapman MH, Xue A, Webster GJ, Clarkson A, Gill A, Norton ID, Smith RC, et al. A combination of serum leucine-rich α-2-glycoprotein 1, CA19-9 and interleukin-6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer. 2011; 105:1370–1378.30. Andersen JD, Boylan KL, Jemmerson R, Geller MA, Misemer B, Harrington KM, Weivoda S, Witthuhn BA, Argenta P, Vogel RI, et al. Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients. J Ovarian Res. 2010; 3:21.31. Dale DC, Fauci AS, Guerry DI, Wolff SM. Comparison of agents producing a neutrophilic leukocytosis in man: hydrocortisone, prednisone, endotoxin, and etiocholanolone. J Clin Invest. 1975; 56:808–813.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta-glucuronidase Activity in Rheumatoid Arthritis and Osteoarthritis
- Serum Interleukin-10 Levels in Rheumatoid Arthritis Patients
- Identification of Human LRG1 Polymorphisms and Their Genetic Association with Rheumatoid Arthritis
- The Study of beta-Glucuronidase Activity in Selected Orthopaedic Disease
- Combination of leucine-rich alpha-2 glycoprotein and fecal markers detect Crohn’s disease activity confirmed by balloon-assisted enteroscopy