J Korean Med Sci.
2009 Feb;24(1):32-39. 10.3346/jkms.2009.24.1.32.
Long-Term Exercise Training Attenuates Age-Related Diastolic Dysfunction: Association of Myocardial Collagen Cross-Linking
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Cardiovascular Center, Cardiovascular Research Institute and Healthcare Research Institute, Seoul National University Hospital, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Cardiovascular Center and Cardiovascular Research Institute, Seoul National University Bundang Hospital, Seongnam, Korea. ross1042@gmail.com
- 3Department of Radiology, Seoul National University College of Medicine, Cardiovascular Center and Cardiovascular Research Institute, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Food and Nutrition, College of Natural Science, Silla University, Busan, Korea.
- KMID: 1794403
- DOI: http://doi.org/10.3346/jkms.2009.24.1.32
Abstract
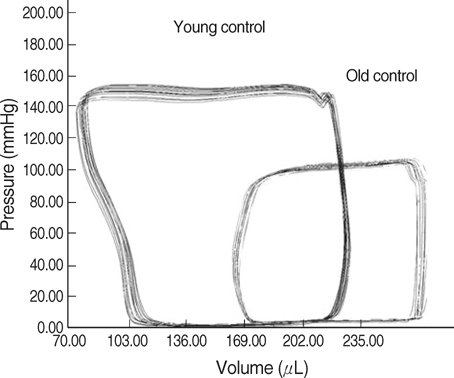
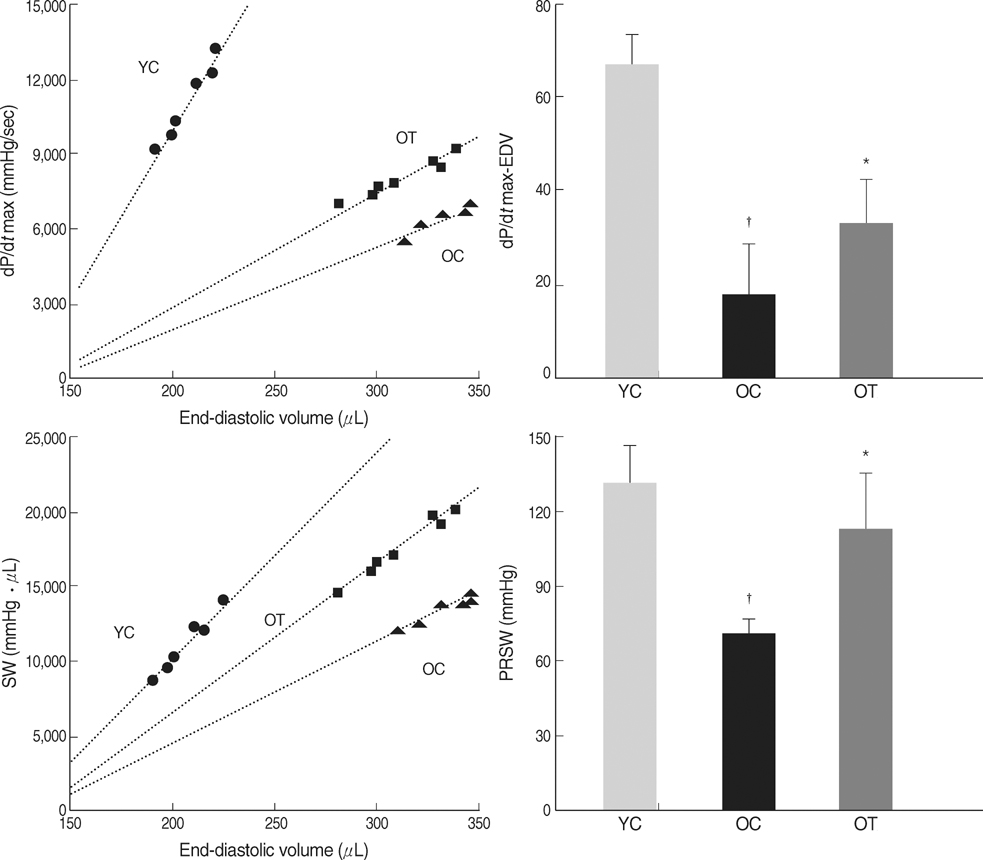
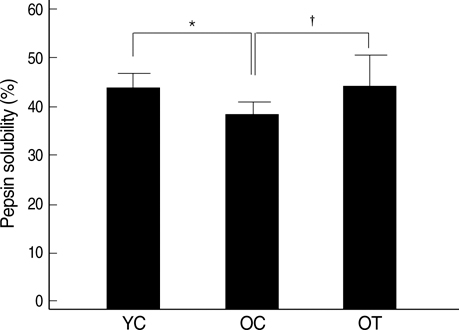
- The incidence of diastolic heart failure increases dramatically with age. We investigated the impact of long-term exercise training on age-related diastolic dysfunction. Old (25-month-old) male Fischer 344 rats were studied after 12 weeks of treadmill exercise training or sedentary cage life (N=7, in each group). We determined cardiac performance using a pressure-volume conductance catheter and magnetic resonance imaging. Collagen volume fraction (CVF) and myocardial collagen solubility by pepsin as an index of advanced glycation end products (AGEs) crosslinked collagen were measured. The maximal slope of systolic pressure increment (+dP/dt) and the slope of end-systolic pressure-volume relation were higher, and end diastolic volume (EDV), delta EDV (the percentage of the EDV increment-to-baseline EDV) and the slope of end-diastolic pressure-volume relation were lower in training group. The maximal slope of diastolic pressure decrement (-dP/dt) and time constant of LV pressure decay (tau) had no difference. AGEs cross-linked collagen, not CVF was reduced by exercise training. Long-term exercise training appears to attenuate age-related deterioration in cardiac systolic function and myocardial stiffness and could be reduce in pathologic AGEs cross-linked collagen in myocardium.
MeSH Terms
Figure
Reference
-
1. Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. The prevalence of left ventricular diastolic filling abnormalities in patients with suspected heart failure. Eur Heart J. 1997. 18:981–984.
Article2. Kitzman DW. Diastolic heart failure in the elderly. Heart Fail Rev. 2002. 7:17–27.
Article3. Downes TR, Nomeir AM, Smith KM, Stewart KP, Little WC. Mechanism of altered pattern of left ventricular filling with aging in subjects without cardiac disease. Am J Cardiol. 1989. 64:523–527.
Article4. Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol. 1987. 10:42A–47A.
Article5. Jyothirmayi GN, Soni BJ, Masurekar M, Lyons M, Regan TJ. Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Ther. 1998. 3:319–326.
Article6. Norton GR, Tsotetsi J, Trifunovic B, Hartford C, Candy GP, Woodiwiss AJ. Myocardial stiffness is attributed to alterations in cross-linked collagen rather than total collagen or phenotypes in spontaneously hypertensive rats. Circulation. 1997. 96:1991–1998.
Article7. Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports. 2005. 15:231–240.
Article8. Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998. 106:1–56.
Article9. Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000. 97:2809–2813.
Article10. Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004. 110:1799–1805.
Article11. Takemoto KA, Bernstein L, Lopez JF, Marshak D, Rahimtoola SH, Chandraratna PA. Abnormalities of diastolic filing of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am Heart J. 1992. 124:143–148.12. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999. 54:B492–B501.
Article13. Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000. 89:1462–1468.
Article14. Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Haskó G, Kollai M, Szabó C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol. 2004. 287:H2132–H2137.
Article15. Woessner JF. The determination of hydroxyproline in tissue and protein samples containing proportions of this amino acid. Arch Biochem Biophys. 1961. 93:440–447.16. Kochakian M, Manjula BN, Egan JJ. Chronic dosing with aminoguanidine and novel advanced glycosylation end product-formation inhibitors ameliorates cross-linking of tail tendon collagen in STZ-induced diabetic rats. Diabetes. 1996. 45:1694–1700.
Article17. Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003. 92:785–792.
Article18. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002. 105:1387–1393.19. Maurer MS, Spevack D, Burkhoff D, Kronzon I. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol. 2004. 44:1543–1549.20. Yamamoto K, Masuyama T, Sakata Y, Nishikawa N, Mano T, Yoshida J, Miwa T, Sugawara M, Yamaguchi Y, Ookawara T, Suzuki K, Hori M. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc Res. 2002. 55:76–82.
Article21. Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993. 88:116–126.
Article22. Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001. 104:221–226.
Article23. Kass DA, Bronzwaer JG, Paulus WJ. What mechanisms underlie diastolic dysfunction in heart failure? Circ Res. 2004. 94:1533–1542.
Article24. Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003. 21:3–12.
Article25. Herrmann KL, McCulloch AD, Omens JH. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am J Physiol Heart Circ Physiol. 2003. 284:H1277–H1284.26. Takala TE, Rämö P, Kiviluoma K, Vihko V, Kainulainen H, Kettunen R. Effects of training and anabolic steroids on collagen synthesis in dog heart. Eur J Appl Physiol Occup Physiol. 1991. 62:1–6.
Article27. Woodiwiss AJ, Oosthuyse T, Norton GR. Reduced cardiac stiffness following exercise is associated with preserved myocardial collagen characteristics in the rat. Eur J Appl Physiol Occup Physiol. 1998. 78:148–154.
Article28. Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW. Glycation, glycoxydation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes. 1994. 43:676–683.29. Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of autoxidative glycosylation in diabetes. Biochem J. 1987. 245:243–250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Left Ventricular Pressure-Volume Relationship and Effect of Long-term Exercise in A Rat Model of Advanced Aging
- Relationship Between Serum Biochemical Markers of Myocardial Fibrosis and Diastolic Function at Rest and With Exercise in Hypertrophic Cardiomyopathy
- The Usefulness of Doppler Tissue Image in Evaluation of Left Ventricular Systolic and Diastolic Dysfunction
- Perioperative management of left ventricular diastolic dysfunction and heart failure: an anesthesiologist's perspective
- Characteristics of Myocardial Deformation and Rotation in Subjects With Diastolic Dysfunction Without Diastolic Heart Failure