Yonsei Med J.
2013 Sep;54(5):1285-1288. 10.3349/ymj.2013.54.5.1285.
IgG4-Related Sclerosing Disease Involving the Superior Vena Cava and the Atrial Septum of the Heart
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. paxco@yuhs.ac
- 3Department of Cardiothoracic Surgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1793181
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1285
Abstract
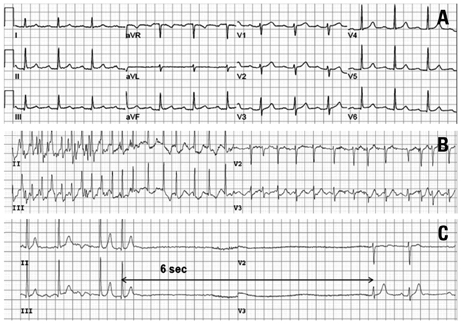
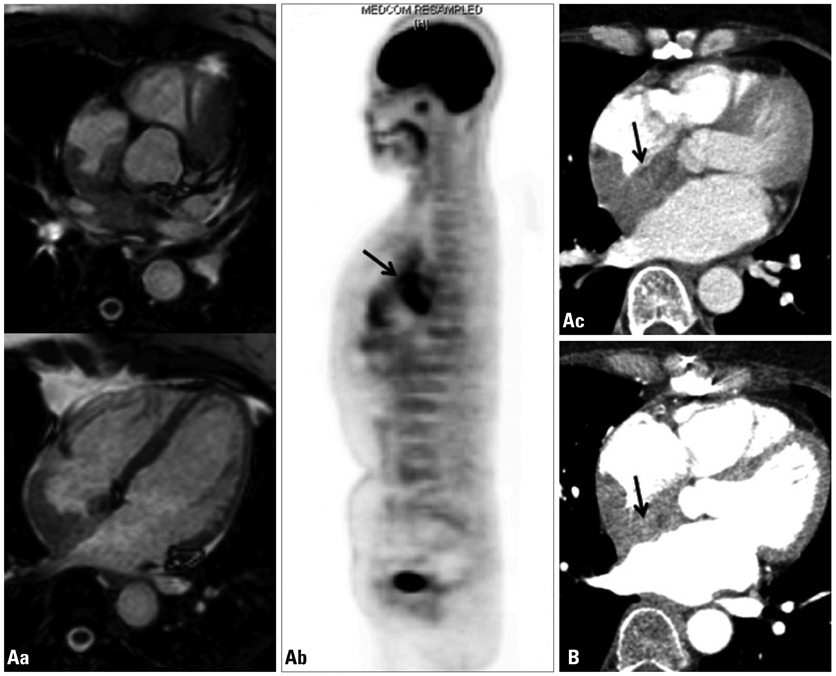
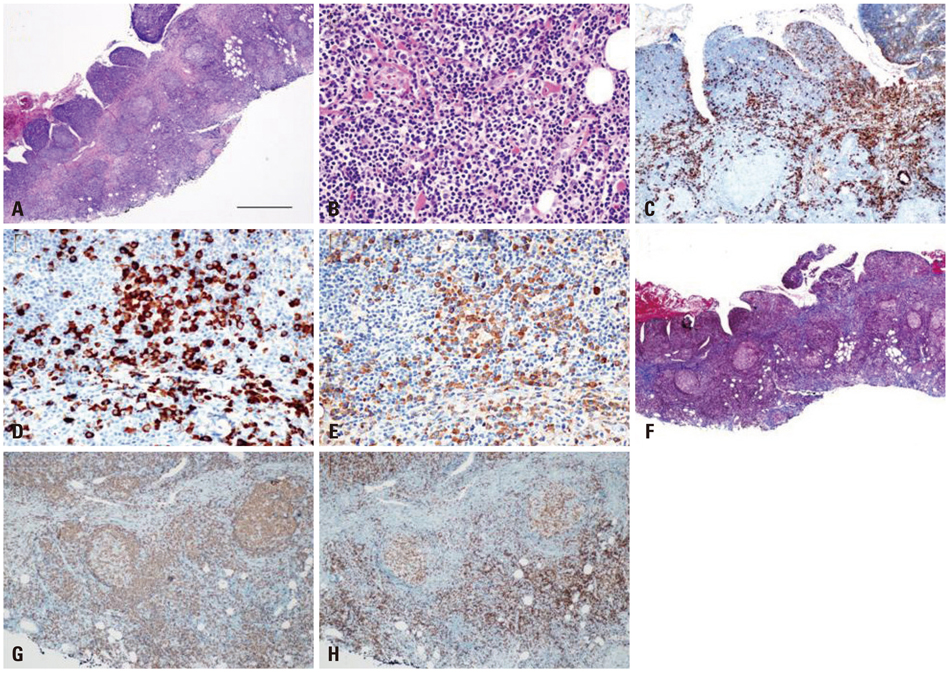
- A 55-year-old woman presented with frequent episodes of syncope due to sinus pauses. During ambulatory Holter monitoring, atrial fibrillation and first-degree atrioventricular nodal block were observed. Magnetic resonance imaging and CT scans showed a tumor-like mass from the superior vena cava to the right atrial septum. Open chest cardiac biopsy was performed. The tumor was composed of proliferating IgG4-positive plasma cells and lymphocytes with surrounding sclerosis. The patient was diagnosed with IgG4-related sclerosing disease. Because of frequent sinus pauses and syncope, a permanent pacemaker was implanted. The cardiac mass was inoperable, but it did not progress during the one-year follow-up.
MeSH Terms
Figure
Reference
-
1. Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001; 344:732–738.
Article2. Cheuk W, Chan JK. IgG4-related sclerosing disease: a critical appraisal of an evolving clinicopathologic entity. Adv Anat Pathol. 2010; 17:303–332.3. Ishizaka N, Sakamoto A, Imai Y, Terasaki F, Nagai R. Multifocal fibrosclerosis and IgG4-related disease involving the cardiovascular system. J Cardiol. 2012; 59:132–138.
Article4. Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006; 4:23.
Article5. Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Yoshikawa J, et al. Immunoglobulin G4-related periaortitis and periarteritis: CT findings in 17 patients. Radiology. 2011; 261:625–633.
Article6. Omura Y, Yoshioka K, Tsukamoto Y, Maeda I, Morikawa T, Konishi Y, et al. Multifocal fibrosclerosis combined with idiopathic retro-peritoneal and pericardial fibrosis. Intern Med. 2006; 45:461–464.
Article7. Baur M, Hulla W, Kienzer H, Klimpfinger M, Dittrich Ch. [Pericarditis as the initial manifestation of retroperitoneal fibrosis--a case report]. Wien Med Wochenschr. 2002; 152:230–232.8. Sakamoto A, Nagai R, Saito K, Imai Y, Takahashi M, Hosoya Y, et al. Idiopathic retroperitoneal fibrosis, inflammatory aortic aneurysm, and inflammatory pericarditis--retrospective analysis of 11 case histories. J Cardiol. 2012; 59:139–146.
Article9. Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Morphological changes after steroid therapy in autoimmune pancreatitis. Scand J Gastroenterol. 2004; 39:1154–1158.
Article10. Kamisawa T, Yoshiike M, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Treating patients with autoimmune pancreatitis: results from a long-term follow-up study. Pancreatology. 2005; 5:234–238.
Article11. Kamisawa T, Okamoto A, Wakabayashi T, Watanabe H, Sawabu N. Appropriate steroid therapy for autoimmune pancreatitis based on long-term outcome. Scand J Gastroenterol. 2008; 43:609–613.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistent Left Superior Vena Cava with Absent Right Superior Vena Cava and Large Atrial Septal Defect in Visceroatrial Situs solitus
- A Case of Absent Right Superior Vena Cava
- A Case of Superior Vena Cava Syndrome
- A Case of Behcet's Disease with Superior Vena Cava Syndrome
- A Case of Persistent Left Superior Vena Cava with Interruption of Inferior Vena Cava