J Korean Med Sci.
2013 Sep;28(9):1293-1301. 10.3346/jkms.2013.28.9.1293.
Podoplanin, alpha-Smooth Muscle Actin or S100A4 Expressing Cancer-Associated Fibroblasts Are Associated with Different Prognosis in Colorectal Cancers
- Affiliations
-
- 1Department of Pathology, Chungbuk National University College of Medicine, Cheongju, Korea.
- 2Department of Surgery, Chungbuk National University College of Medicine, Cheongju, Korea.
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. smpark@chungbuk.ac.kr
- KMID: 1793043
- DOI: http://doi.org/10.3346/jkms.2013.28.9.1293
Abstract
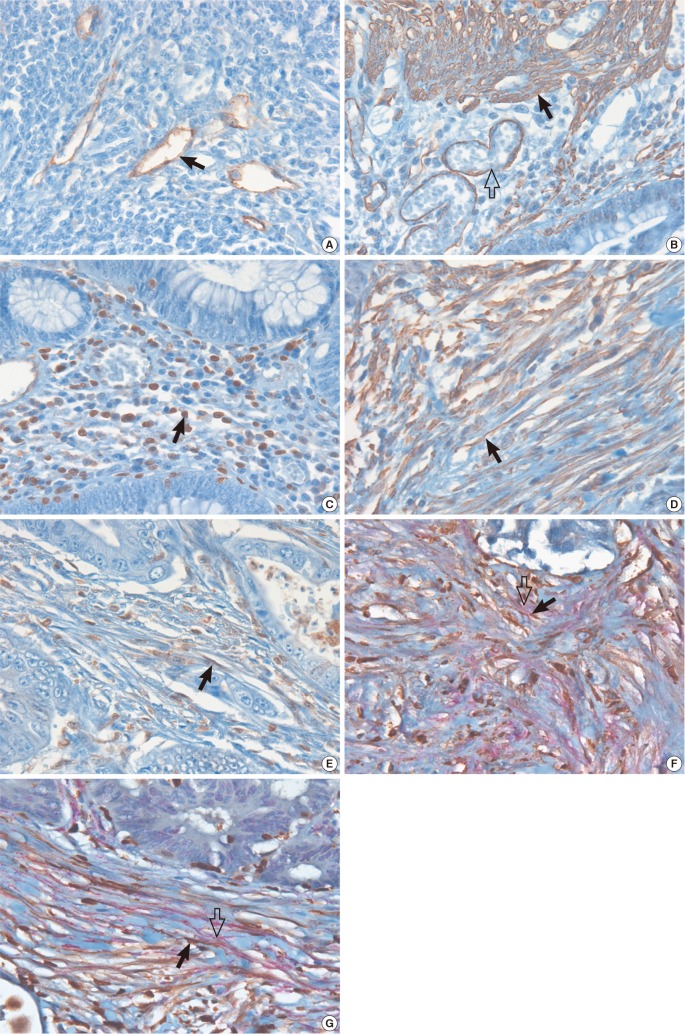
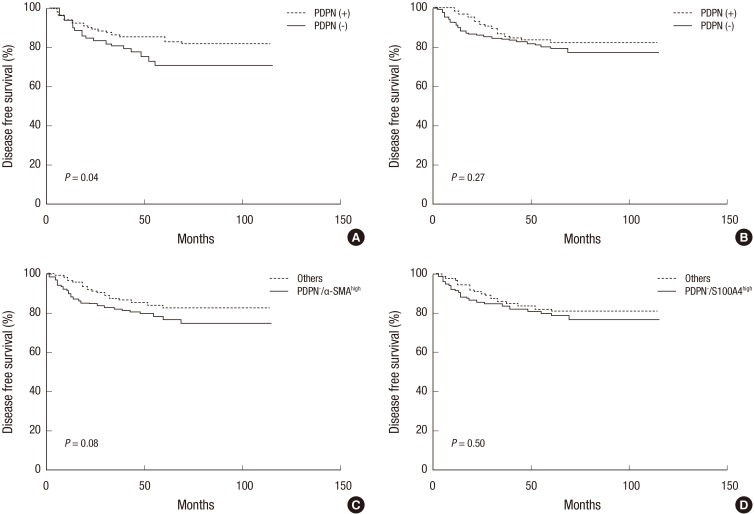
- The interactions between the tumor microenvironment and tumor cells determine the behavior of the primary tumors. Whether cancer-associated fibroblasts (CAF) have a tumor progressive or a protective role likely depends on the type of tumor cells and the CAF subpopulation. In the present study, we analyzed the prognostic significance of CAF subpopulations in colorectal cancer (CRC). CAF phenotypes were analyzed in 302 CRC patients by using antibodies against podoplanin (PDPN), alpha-smooth muscle actin (alpha-SMA), and S100A4. The relationship between the CAF phenotypes and 11 clinicopathological parameters were evaluated and their prognostic significance was analyzed from the disease-free and overall survival times. We observed that at the tumor invasive front, PDPN CAFs were present in 40% of the cases, and S100A4 or alpha-SMA CAFs were detected in all the cases. PDPN/S100A4 and alpha-SMA/S100A4 dual-stained CAFs were observed in 10% and 40% of the cases, respectively. The PDPN+ CAFs were associated with 6 favorable clinicopathological parameters and prolonged disease-free survival time. The PDPN-/alpha-SMA(high) CAFs were associated with 6 aggressive clinicopathological parameters and tended to exhibit shorter disease-free survival time. On the other hand, the PDPN-/S100A4(high) CAFs were associated with 2 tumor progression parameters, but not with disease prognosis. The PDPN+ CAF phenotype is distinct from the alpha-SMA or S100A4 CAFs in that it is associated with less aggressive tumors and a favorable prognosis, whereas the PDPN-/alpha-SMA(high) or PDPN-/S100A4(high) CAFs are associated with tumor progression in CRC. These findings suggest that CAFs can be a useful prognostic biomarker or potential targets of anti-cancer therapy in CRC.
Keyword
MeSH Terms
-
Actins/immunology/*metabolism
Adult
Aged
Aged, 80 and over
Antibodies/immunology
Carcinoembryonic Antigen/blood
Colorectal Neoplasms/*diagnosis/mortality/pathology
Disease-Free Survival
Female
Fibroblasts/cytology/metabolism
Humans
Immunohistochemistry
Lymphatic Metastasis
Male
Membrane Glycoproteins/immunology/*metabolism
Middle Aged
Neoplasm Staging
Phenotype
Prognosis
S100 Proteins/immunology/*metabolism
Tumor Markers, Biological/metabolism
Actins
Antibodies
Carcinoembryonic Antigen
Membrane Glycoproteins
S100 Proteins
Tumor Markers, Biological
Figure
Reference
-
1. Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010; 316:1324–1331. PMID: 20211171.2. Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007; 13:2082–2090. PMID: 17404090.3. Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007; 96:986–992. PMID: 17325702.4. Cheng JD, Dunbrack RL Jr, Valianou M, Rogatko A, Alpaugh RK, Weiner LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002; 62:4767–4772. PMID: 12183436.5. Grégoire M, Lieubeau B. The role of fibroblasts in tumor behavior. Cancer Metastasis Rev. 1995; 14:339–350. PMID: 8821094.6. Martin MS, Caignard A, Hammann A, Pelletier H, Martin F. An immunohistological study of cells infiltrating progressive and regressive tumors induced by two variant subpopulations of a rat colon cancer cell line. Int J Cancer. 1987; 40:87–93. PMID: 3298078.7. Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983; 49:636–649. PMID: 6317982.8. Kono T, Shimoda M, Takahashi M, Matsumoto K, Yoshimoto T, Mizutani M, Tabata C, Okoshi K, Wada H, Kubo H. Immunohistochemical detection of the lymphatic marker podoplanin in diverse types of human cancer cells using a novel antibody. Int J Oncol. 2007; 31:501–508. PMID: 17671675.9. Kitano H, Kageyama S, Hewitt SM, Hayashi R, Doki Y, Ozaki Y, Fujino S, Takikita M, Kubo H, Fukuoka J. Podoplanin expression in cancerous stroma induces lymphangiogenesis and predicts lymphatic spread and patient survival. Arch Pathol Lab Med. 2010; 134:1520–1527. PMID: 20923309.10. Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009; 77:53–62. PMID: 19556810.11. Schulte J, Weidig M, Balzer P, Richter P, Franz M, Junker K, Gajda M, Friedrich K, Wunderlich H, Östman A, et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem Cell Biol. 2012; 138:847–860. PMID: 22820858.12. Vered M, Dayan D, Yahalom R, Dobriyan A, Barshack I, Bello IO, Kantola S, Salo T. Cancer-associated fibroblasts and epithelial-mesenchymal transition in metastatic oral tongue squamous cell carcinoma. Int J Cancer. 2010; 127:1356–1362. PMID: 20340130.13. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998; 58:5248–5257. PMID: 9823339.14. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–268. PMID: 14970275.15. American Joint Committee on Cancer. Manual for staging of cancer. 7th ed. Berlin: Springer;2009.16. Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, Hyland J, O'Donoghue D, Sheahan K. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009; 33:134–141. PMID: 18971777.17. Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, Chen WT, Cheng JD. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007; 13:1736–1741. PMID: 17363526.18. McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007; 39:666–671. PMID: 17196874.19. Nakayama H, Enzan H, Miyazaki E, Naruse K, Kiyoku H, Hiroi M. The role of myofibroblasts at the tumor border of invasive colorectal adenocarcinomas. Jpn J Clin Oncol. 1998; 28:615–620. PMID: 9839502.20. Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, Tollenaar RA. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007; 29:387–398. PMID: 17726261.21. Kawase A, Ishii G, Nagai K, Ito T, Nagano T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K, et al. Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer. 2008; 123:1053–1059. PMID: 18546264.22. Tong L, Yuan S, Feng F, Zhang H. Role of podoplanin expression in esophageal squamous cell carcinoma: a retrospective study. Dis Esophagus. 2012; 25:72–80. PMID: 21895849.23. Foschini MP, Leonardi E, Eusebi LH, Farnedi A, Poli T, Tarsitano A, Cocchi R, Marchetti C, Gentile L, Sesenna E, et al. Podoplanin and E-cadherin expression in preoperative incisional biopsies of oral squamous cell carcinoma is related to lymph node metastases. Int J Surg Pathol. 2013; 21:133–141. PMID: 23349470.24. Schoppmann SF, Berghoff A, Dinhof C, Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H, Birner P. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012; 134:237–244. PMID: 22350732.25. Dumoff KL, Chu CS, Harris EE, Holtz D, Xu X, Zhang PJ, Acs G. Low podoplanin expression in pretreatment biopsy material predicts poor prognosis in advanced-stage squamous cell carcinoma of the uterine cervix treated by primary radiation. Mod Pathol. 2006; 19:708–716. PMID: 16528371.26. Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006; 5:1640–1646. PMID: 17106243.27. Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010; 176:528–535. PMID: 20019188.28. Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth: bystanders turning into key players. Curr Opin Genet Dev. 2009; 19:67–73. PMID: 19211240.29. Pino MS, Kikuchi H, Zeng M, Herraiz MT, Sperduti I, Berger D, Park DY, Iafrate AJ, Zukerberg LR, Chung DC. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology. 2010; 138:1406–1417. PMID: 20026115.30. Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012; 11:257–266. PMID: 22323494.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of cGMP on Smooth Muscle alpha-Actin Expression in Cultured Human Corporal Smooth Muscle Cells
- Effect of Pregnancy on Knee Joint Contracture in the Rat
- The Effects of Tranilast gamma on the Cellular Proliferation and alpha-Smooth Muscle Actin Expression in the Cultured Keratocytes of Rabbit
- The Effect of Amniotic Membrane Graft on the Inhibition of Corneal Opacity After Traumatic Injury
- Change of alpha-SM Actin Expression Induced by the Antibody for TGF-beta in Fibroblast NIH3T3 Culture: The basic research for the inhibition of wound contracture