J Korean Med Sci.
2010 Jul;25(7):1066-1070. 10.3346/jkms.2010.25.7.1066.
Epithelial-Mesenchymal Transitions of Bile Duct Epithelial Cells in Primary Hepatolithiasis
- Affiliations
-
- 1Hepatobiliary Surgery Institute, Southwest Hospital, Third Military Medical University, Chongqing, China. sgwang90@yahoo.com
- 2Affiliated Hospital of Zunyi Medical College, Zunyi, China.
- 3Department of General Surgery, The 324th Hospital of PLA, Chongqing, China.
- KMID: 1792958
- DOI: http://doi.org/10.3346/jkms.2010.25.7.1066
Abstract
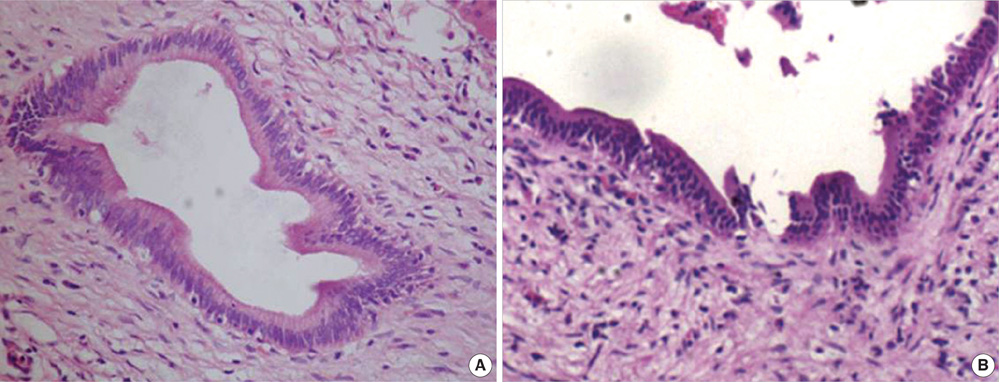
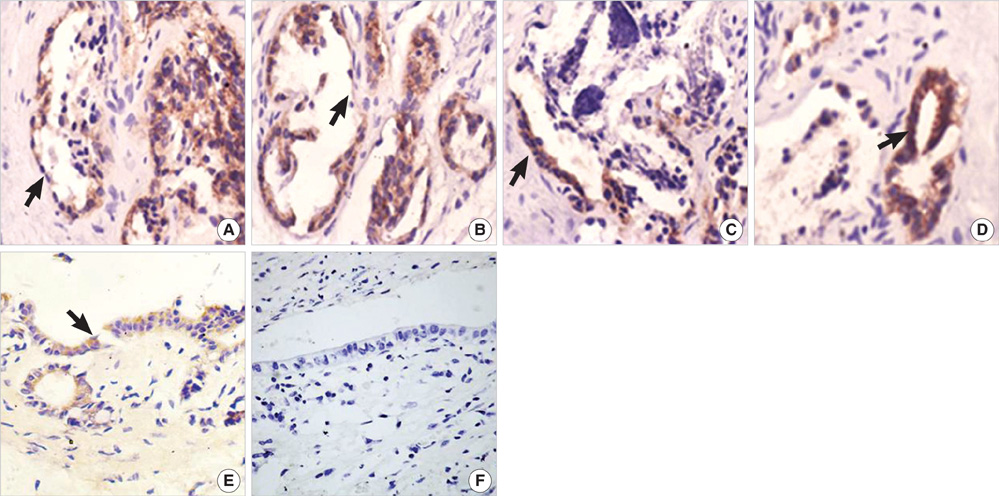
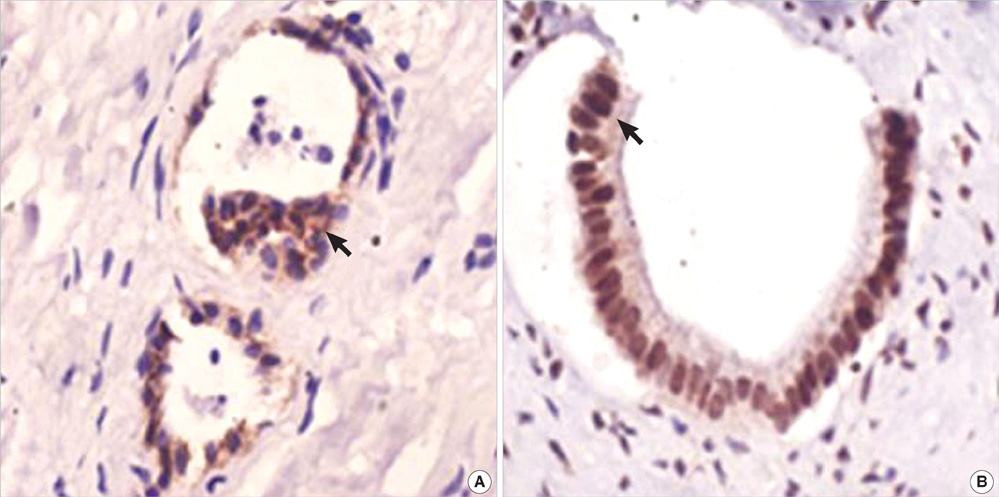
- The purpose of this study was to explore the role of epithelial-mesenchymal transition in the pathogenesis of hepatolithiasis. Thirty-one patients with primary hepatolithiasis were enrolled in this study. Expressions of E-cadherin, alpha-catenin, alpha-SMA, vimentin, S100A4, TGF-beta1 and P-smad2/3 in hepatolithiasis bile duct epithelial cells were examined by immunohistochemistry staining. The results showed that the expressions of the epithelial markers E-cadherin and alpha-catenin were frequently lost in hepatolithiasis (32.3% and 25.9% of cases, respectively), while the mesenchymal markers vimentin, alpha-SMA and S100A4 were found to be present in hepatolithiasis (35.5%, 29.0%, and 32.3% of cases, respectively). The increased mesenchymal marker expression was correlated with decreased epithelial marker expression. The expressions of TGF-beta1 and P-smad2/3 in hepatolithiasis were correlated with the expression of S100A4. These data indicate that TGF-beta1-mediated epithelial-mesenchymal transition might be involved in the formation of hepatolithiasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Shoda J, Tanaka N, Osuga T. Hepatolithiasis-epidemiology and pathogenesis update. Front Biosci. 2003. 8.
Article2. Kubo S, Kinoshita H, Hirohashi K, Hamba H. Hepatolithiasis associated with cholangiocarcinoma. World J Surg. 1995. 19:637–641.
Article3. Nakai A, Imano M, Takeyama Y, Shiozaki H, Ohyanagi H. An immunohistochemical study of osteopontin in hepatolithiasis. J Hepatobil Pancreat Surg. 2008. 15:615–621.
Article4. Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002. 2:442–454.
Article5. Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant. 2005. 5:1367–1374.
Article6. Robertson H, Kirby JA, Yip WW, Jones DE, Burt AD. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007. 45:977–981.
Article7. Schiffer M, von Gersdorff G, Bitzer M, Susztak K, Bottinger EP. Smad proteins and transforming growth factor-beta signaling. Kidney Int. 2000. 77:Suppl. S45–S52.8. ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004. 29:265–273.9. Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008. 88:112–123.
Article10. Han DB, Dong JH, Guo GJ. Evaluation of our clinicopathologic stage for 1259 patients with intrahepatic stones. Acta Academiae Medicinae Militaris Tertiae. 2006. 28:1337–1338.11. Asayama Y, Taguchi Ki K, Aishima Si S, Nishi H, Masuda K, Tsuneyoshi M. The mode of tumour progression in combined hepatocellular carcinoma and cholangiocarcinoma: an immunohistochemical analysis of E-cadherin, alpha-catenin and beta-catenin. Liver. 2002. 22:43–50.
Article12. Nakajima S, Doi R, Toyoda E, Tsuji S, Wada M, Koizumi M, Tulachan SS, Ito D, Kami K, Mori T, Kawaguchi Y, Fujimoto K, Hosotani R, Imamura M. N-cadherin expression and epithelial-mesenchymal transition in pancreatic carcinoma. Clin Cancer Res. 2004. 10:4125–4133.
Article13. Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004. 118:277–279.14. Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007. 31:479–482.
Article15. Inayoshi J, Ichida T, Sugitani S, Tsuboi Y, Genda T, Honma N, Asakura H. Gross appearance of hepatocellular carcinoma reflects E-cadherin expression and risk of early recurrence after surgical treatment. J Gastroenterol Hepatol. 2003. 18:673–677.
Article16. Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004. 117:927–939.
Article17. Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001. 159:1313–1321.18. Choi HS, Savard CE, Choi JW, Kuver R, Lee SP. Paclitaxel interrupts TGF-beta1 signaling between gallbladder epithelial cells and myofibroblasts. J Surg Res. 2007. 141:183–191.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Observation of Histologic Changes of Major Intrahepatic Bile Duct Epithelium in the Resected Liver Tissue with Hepatolithiasis
- Expression of Proliferating Cell Nuclear Antigen (PCNA) of Major Intrahepatic Bile Duct Epithelium in Resected Liver Tissue with Hepatolithiasis and Hepatolithiasis Associated with Cholangiocarcinoma
- The Crucial Role of Cholangiocytes in Cholangiopathies
- The Surgical Treatments for the Hepatolithiasis
- Histochemistry of Six Lectins in the Tissues of the Flat Fish Paralichthys olivaceus