Korean J Gastroenterol.
2009 Jul;54(1):20-27. 10.4166/kjg.2009.54.1.20.
Therapeutic Effect of Allogenic Bone Marrow Transplantation in Acute TNBS-induced Colitis
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. chs@catholic.ac.kr
- 2Department of Pathology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 1792762
- DOI: http://doi.org/10.4166/kjg.2009.54.1.20
Abstract
- BACKGROUND/AIMS
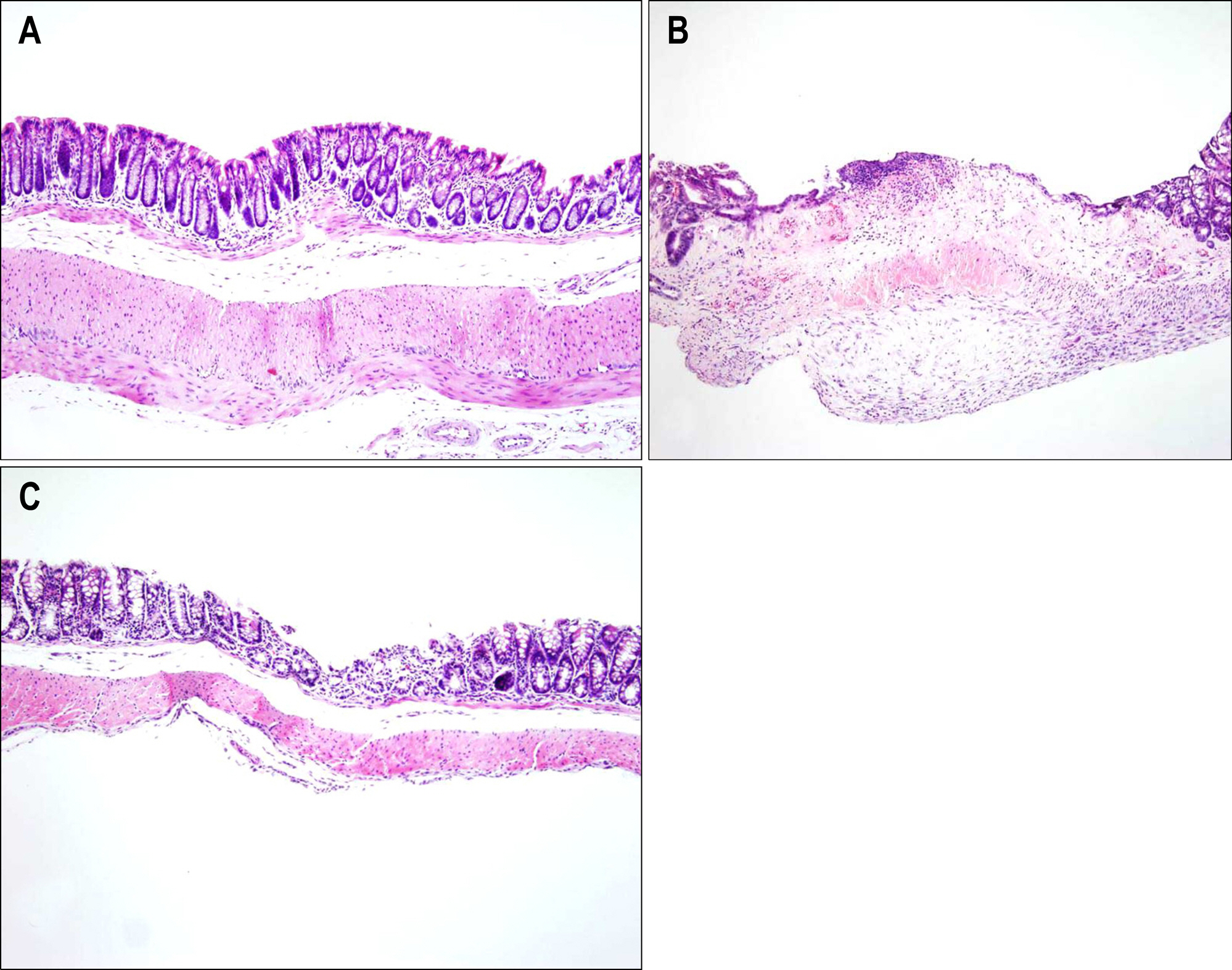
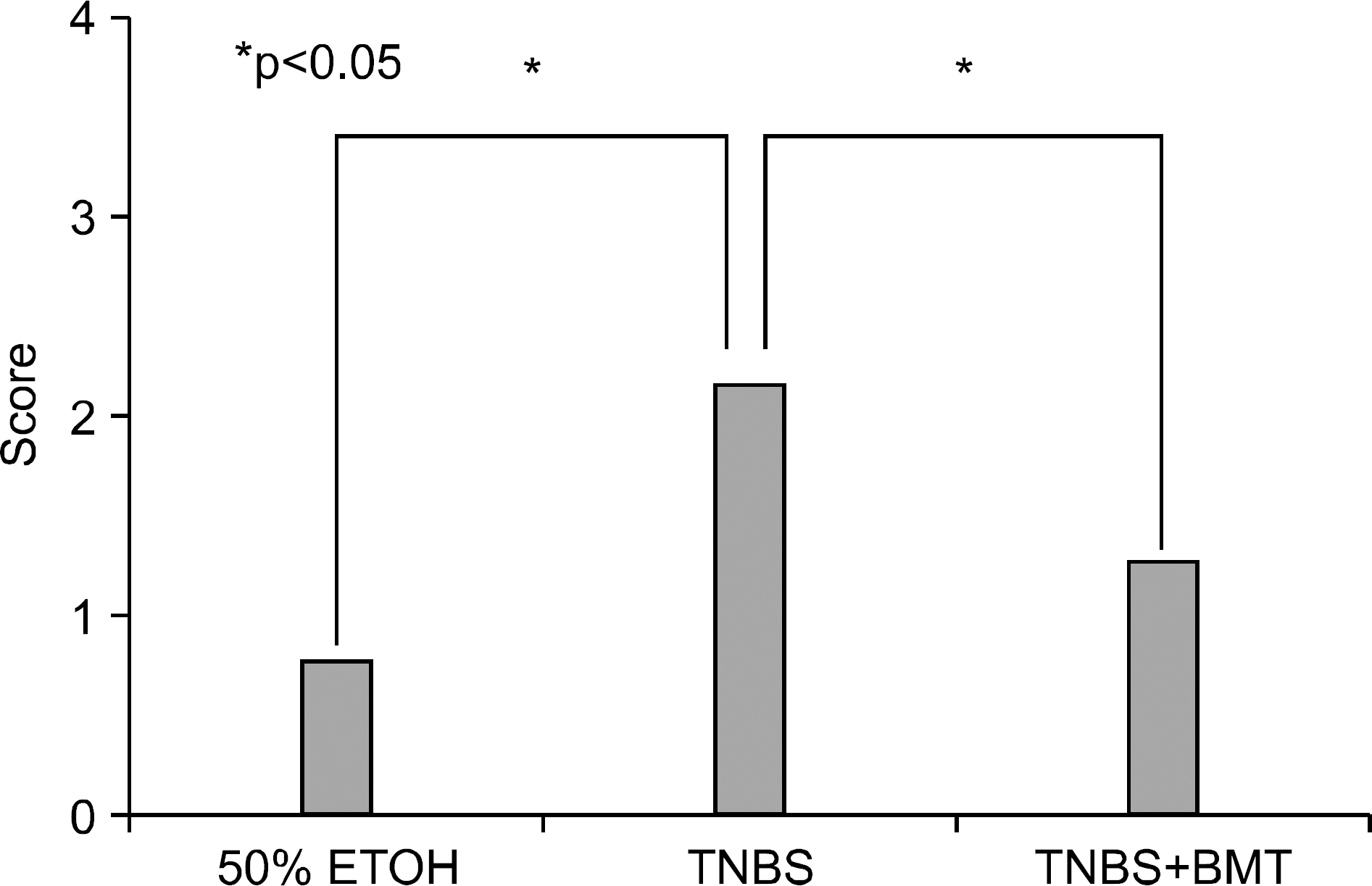
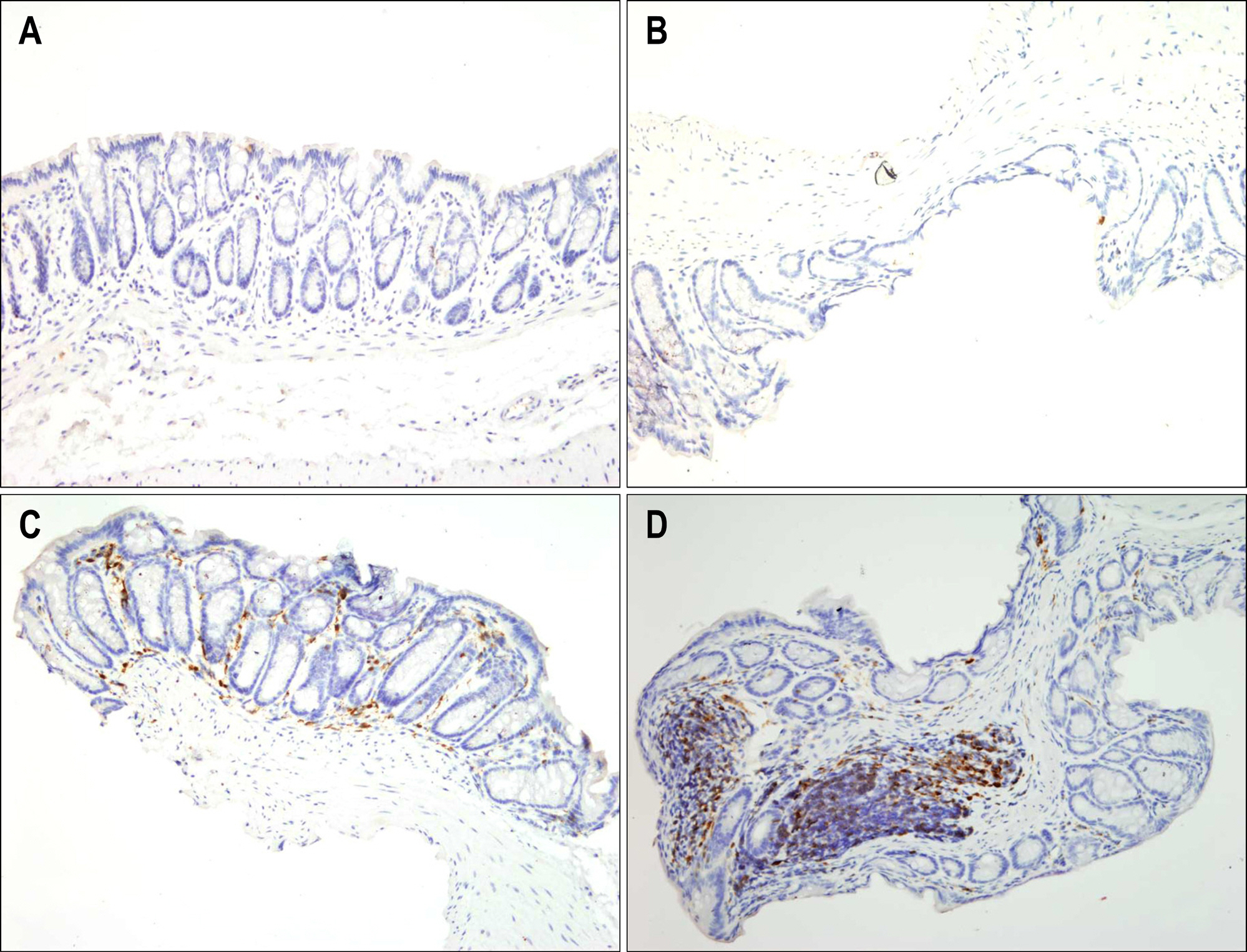
Bone marrow-derived cells (BMDC) contribute to tissue maintenance under many kinds of pathologic conditions. We carried out a study to see how BMDC play a role in the treatment of experimental murine colitis. METHODS: We divided the animals into 3 groups and treated them with 50% ethanol (control group), 2,4,6-trinitrobenzene sulfinic acid colitis (TNBS group), and TNBS+bone marrow transplant (BMT group). To induce colitis, TNBS (5.0 mg/mouse) dissolved in 50% ethanol was injected into anus weekly for two weeks. Bone marrow transplantations were performed using bone marrow of male transgenic mouse (donor) with green fluoresence protein (GFP) into female wild type mouse (recipient) three weeks before TNBS instillation. All animals were sacrificed, and colons were extracted one week after the last TNBS instillation. We measured microscopic scores of mucosal injury and investigated the GFP expression for bone marrow engraftment. The immunostaining of vimentin and alpha-smooth muscle actin (alpha-SMA) for myofibroblasts was performed. RESULTS: The score of mucosal injury in the TNBS group was much more severe than those in control, and reduced significantly by BMT (p<0.05). GFP-positive cells were almost deposited in pericryptal niche of BMT group but not at all in both control and TNBS group. Most of myofibroblasts stained with both vimentin and SMA also infiltrated into pericryptal niche. But, the number of myofibroblasts stained with vimentin and SMA in both control and TNBS group was smaller than that in BMT group. CONCLUSIONS: BMDC deposited on pericryptal niche might have a significant role in repairing acute experimental murine colitis.
MeSH Terms
Figure
Reference
-
1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007; 369:1641–1657.
Article2. Katz JA. Management of inflammatory bowel disease in adults. J Dig Dis. 2007; 8:65–71.
Article3. Garcí a-Olmo D, Garcí a-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase 1 clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005; 48:1416–1423.4. Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005; 128:552–563.
Article5. Talbot DC, Montes A, Teh WL, Nandi A, Powles RL. Remission of Crohn's disease following allogeneic bone marrow transplant for acute leukemia. Hosp Med. 1998; 59:580–581.6. Herzog EL, Krause DS. Engraftment of marrow-derived epithelial cells: the role of fusion. Proc Am Thorac Soc. 2006; 3:691–695.
Article7. Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002; 8:1011–1017.
Article8. Okamoto R, Watanabe M. Prospects for regeneration of gastrointestinal epithelia using bone-marrow cells. Trends Mol Med. 2003; 9:286–290.
Article9. Borue X, Lee S, Grove J, et al. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004; 165:1767–1772.
Article10. Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005; 13:109–118.
Article11. Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005; 40:1089–1099.
Article12. Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007; 132:944–954.
Article13. Bamba S, Lee CY, Brittan M, et al. Bone marrow transplantation ameliorates pathology in interleukin-10 knockout colitic mice. J Pathol. 2006; 209:265–273.
Article14. Brittan M, Hunt T, Jeffery R, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002; 50:752–757.
Article15. Powell DW. Myofibroblasts: paracrine cells important in health and disease. Trans Am Clin Climatol Assoc. 2000; 111:271–292.16. Brittan M, Chance V, Elia G, et al. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005; 128:1984–1995.
Article17. Brittan M, Wright NA. Stem cell in gastrointestinal structure and neoplastic development. Gut. 2004; 53:899–910.
Article18. Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999; 277:C183–C201.
Article19. Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002; 12:502–508.
Article20. Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006; 420:339–361.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Eosinophilic Folliculitis after Allogenic Bone Marrow Transplantatino in Acute Myelogenous Leukemia
- The Case Report of a Child with High-Risk Acute Lymphoblastic Leukemia, Treated with Allogenic Peripheral Blood Stem Cell Transplantation
- Sequential changes of bone marrow pathology and BFU-E in recipients of allogenic bone marrow transplantation
- Allogenic bone marrow transplantation in rabbit
- Saccharomyces boulardii Reduced Intestinal Inflammation in Mice Model of 2,4,6-trinitrobencene Sulfonic Acid Induced Colitis: Based on Microarray