Korean J Ophthalmol.
2014 Apr;28(2):170-176. 10.3341/kjo.2014.28.2.170.
Effect of Macrophage Migration Inhibitory Factor on Corneal Sensitivity after Laser In Situ Keratomileusis in Rabbit
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. jyhyon@snu.ac.kr
- 2Department of Ophthalmology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
- 3CNRS UMR 5160, CRLC, Montpellier, France.
- 4Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Palm Beach Gardens, FL, USA.
- KMID: 1792112
- DOI: http://doi.org/10.3341/kjo.2014.28.2.170
Abstract
- PURPOSE
To investigate the effect of macrophage migration inhibitory factor (MIF) on corneal sensitivity after laser in situ keratomileusis (LASIK) surgery.
METHODS
New Zealand white rabbits were used in this study. A hinged corneal flap (160-microm thick) was created with a microkeratome, and -3.0 diopter excimer laser ablation was performed. Expressions of MIF mRNA in the corneal epithelial cells and surrounding inflammatory cells were analyzed using reverse transcription polymerase chain reaction at 48 hours after LASIK. After LASIK surgery, the rabbits were topically given either 1) a balanced salt solution (BSS), 2) MIF (100 ng/mL) alone, or 3) a combination of nerve growth factor (NGF, 100 ug/mL), neurotrophine-3 (NT-3, 100 ng/mL), interleukin-6 (IL-6, 5 ng/mL), and leukemia inhibitory factor (LIF, 5 ng/mL) four times a day for three days. Preoperative and postoperative corneal sensitivity at two weeks and at 10 weeks were assessed using the Cochet-Bonnet esthesiometer.
RESULTS
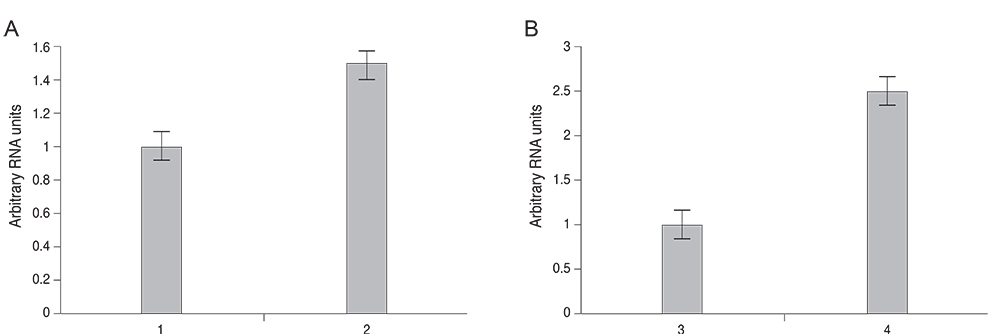
Expression of MIF mRNA was 2.5-fold upregulated in the corneal epithelium and 1.5-fold upregulated in the surrounding inflammatory cells as compared with the control eyes. Preoperative baseline corneal sensitivity was 40.56 +/- 2.36 mm. At two weeks after LASIK, corneal sensitivity was 9.17 +/- 5.57 mm in the BSS treated group, 21.92 +/- 2.44 mm in the MIF treated group, and 22.42 +/- 1.59 mm in the neuronal growth factors-treated group (MIF vs. BSS, p < 0.0001; neuronal growth factors vs. BSS, p < 0.0001; MIF vs. neuronal growth factors, p = 0.815). At 10 weeks after LASIK, corneal sensitivity was 15.00 +/- 9.65, 35.00 +/- 5.48, and 29.58 +/- 4.31 mm respectively (MIF vs. BSS, p = 0.0001; neuronal growth factors vs. BSS, p = 0.002; MIF vs. neuronal growth factors, p = 0.192). Treatment with MIF alone could achieve as much of an effect on recovery of corneal sensation as treatment with combination of NGF, NT-3, IL-6, and LIF.
CONCLUSIONS
Topically administered MIF plays a significant role in the early recovery of corneal sensitivity after LASIK in the experimental animal model.
Keyword
MeSH Terms
-
Animals
Epithelium, Corneal/*drug effects/innervation/physiology
Female
Humans
Interleukin-6/pharmacology
Keratomileusis, Laser In Situ/*methods
Leukemia Inhibitory Factor/pharmacology
Macrophage Migration-Inhibitory Factors/genetics/*pharmacology
Models, Animal
Nerve Growth Factor/pharmacology
Nerve Regeneration/*drug effects/physiology
Neurotrophin 3/pharmacology
RNA, Messenger/metabolism
Rabbits
Recovery of Function/*drug effects/physiology
Sensation/*drug effects/physiology
Interleukin-6
Leukemia Inhibitory Factor
Macrophage Migration-Inhibitory Factors
Nerve Growth Factor
Neurotrophin 3
RNA, Messenger
Figure
Reference
-
1. Lee BH, McLaren JW, Erie JC, et al. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002; 43:3660–3664.2. Chaudhary S, Namavari A, Yco L, et al. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea. 2012; 31:1460–1467.3. Esquenazi S, Bazan HE, Bui V, et al. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005; 46:3121–3127.4. Yang P, Wen H, Ou S, et al. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol. 2012; 236:19–27.5. Nathan CF, Karnovsky ML, David JR. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971; 133:1356–1376.6. Matsuda A, Tagawa Y, Matsuda H, Nishihira J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996; 385:225–228.7. Bacher M, Meinhardt A, Lan HY, et al. MIF expression in the rat brain: implications for neuronal function. Mol Med. 1998; 4:217–230.8. Nishibori M, Nakaya N, Tahara A, et al. Presence of macrophage migration inhibitory factor (MIF) in ependyma, astrocytes and neurons in the bovine brain. Neurosci Lett. 1996; 213:193–196.9. Matsunaga J, Sinha D, Pannell L, et al. Enzyme activity of macrophage migration inhibitory factor toward oxidized catecholamines. J Biol Chem. 1999; 274:3268–3271.10. Matsunaga J, Sinha D, Solano F, et al. Macrophage migration inhibitory factor (MIF): its role in catecholamine metabolism. Cell Mol Biol (Noisy-le-grand). 1999; 45:1035–1040.11. Nishio Y, Minami A, Kato H, et al. Identification of macrophage migration inhibitory factor (MIF) in rat peripheral nerves: its possible involvement in nerve regeneration. Biochim Biophys Acta. 1999; 1453:74–82.12. Nishio Y, Nishihira J, Ishibashi T, et al. Role of macrophage migration inhibitory factor (MIF) in peripheral nerve regeneration: anti-MIF antibody induces delay of nerve regeneration and the apoptosis of Schwann cells. Mol Med. 2002; 8:509–520.13. Matsuda A, Tagawa Y, Matsuda H, Nishihira J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996; 385:225–228.14. Oh SY, Choi JS, Kim EJ, et al. The role of macrophage migration inhibitory factor in ocular surface disease pathogenesis after chemical burn in the murine eye. Mol Vis. 2010; 16:2402–2411.15. Tsujimura H, Tamura T, Gongora C, et al. ICSBP/IRF-8 retrovirus transduction rescues dendritic cell development in vitro. Blood. 2003; 101:961–969.16. Kuwata T, Gongora C, Kanno Y, et al. Gamma interferon triggers interaction between ICSBP (IRF-8) and TEL, recruiting the histone deacetylase HDAC3 to the interferon-responsive element. Mol Cell Biol. 2002; 22:7439–7448.17. Joo MJ, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004; 122:1338–1341.18. Matsuda A, Tagawa Y, Matsuda H, Nishihira J. Expression of macrophage migration inhibitory factor in corneal wound healing in rats. Invest Ophthalmol Vis Sci. 1997; 38:1555–1562.19. Heigle TJ, Pflugfelder SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996; 15:135–138.20. Müller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997; 38:985–994.21. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–589.22. Koh SW. Ciliary neurotrophic factor released by corneal endothelium surviving oxidative stress ex vivo. Invest Ophthalmol Vis Sci. 2002; 43:2887–2896.23. You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000; 41:692–702.24. Zagon IS, Sassani JW, McLaughlin PJ. Reepithelialization of the human cornea is regulated by endogenous opioids. Invest Ophthalmol Vis Sci. 2000; 41:73–81.25. Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001; 108:1230–1235.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Nerve Growth Factor on Corneal Sensitivity after Laser in Situ Keratomileusis
- Changes in Corneal Sensitivity after Laser in-situ Keratom ileusis
- Surgical treatment of presbyopia
- Corneal Keloid Case after Laser-assisted in-situ Keratomileusis Surgery in Korea
- Corneal Endothelial Damage after Deep Excimer Laser Ablation