Korean J Ophthalmol.
2013 Dec;27(6):416-420. 10.3341/kjo.2013.27.6.416.
The Pathologic Characteristics of Pingueculae on Autofluorescence Images
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University Hospital, Seoul, Korea. jck50ey@kornet.net
- KMID: 1792076
- DOI: http://doi.org/10.3341/kjo.2013.27.6.416
Abstract
- PURPOSE
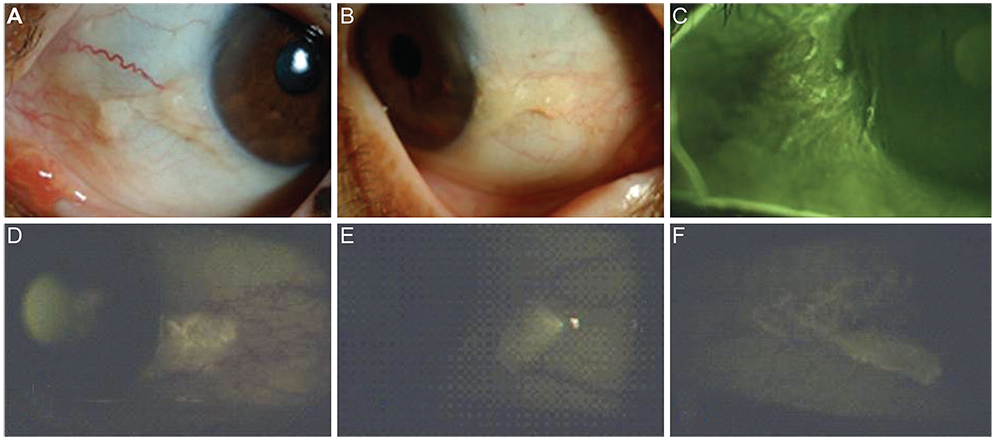
To analyze the autofluorescence (AF) properties of pinguecula using cobalt-blue and yellow filters and to investigate the nature and pathogenesis of pingueculae using histochemical and immunohistochemical staining.
METHODS
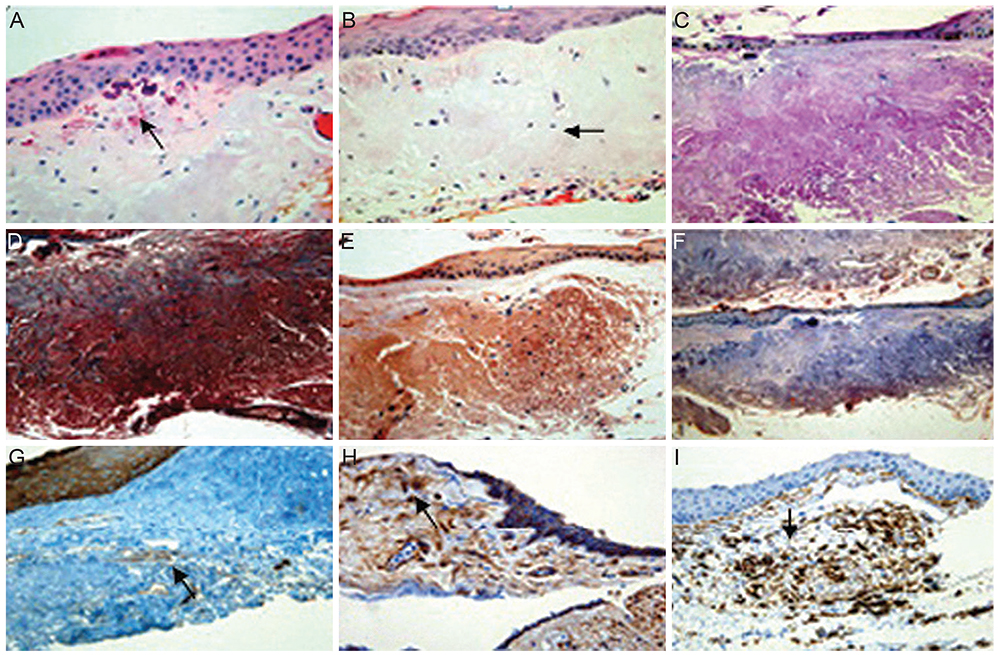
Fifty pingueculae in 40 patients were included in this study. AF of the pingueculae was observed and analyzed using a cobalt-blue filter with an additional yellow filter on a slit-lamp. Hematoxylin-eosin and immunohistochemical stainings were performed on surgical specimens of pingueculae that were prepared from each patient. Immunohistochemical staining included Congo red, Oil Red O, periodic acid-Schiff (PAS), Masson's trichrome, transglutaminase-2 (TG-2), mesenchymal stem cell markers CD29 (beta-1-integrin), and CD34.
RESULTS
AF images revealed hyper-AF in the pinguecula area. The AF lesions of pingueculae showed superficial punctuate erosions and avascular lesions. Deposition of eosinophilic and amorphous materials in the subepithelial layer of the pinguecula were observed on hematoxylin-eosin staining. Historeactivities to Congo red, PAS, Oil Red O, alcian blue, and Masson's trichrome were not detected, but immunoreactivities to CD29, CD34, and TG-2 were detected in the pingueculae with AF. However, CD29, CD34, and TG-2 were not detected in the pingueculae without AF.
CONCLUSIONS
The AF of pingueculae may be related to CD29, CD34, and TG-2. We suggest that pingueculae with AF have a different pathogenesis compared to pingueculae without AF.
Keyword
MeSH Terms
Figure
Reference
-
1. Young JD, Finlay RD. Primary spheroidal degeneration of the cornea in Labrador and northern Newfoundland. Am J Ophthalmol. 1975; 79:129–134.2. Dushku N, Reid TW. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr Eye Res. 1997; 16:1179–1192.3. Dong N, Li W, Lin H, et al. Abnormal epithelial differentiation and tear film alteration in pinguecula. Invest Ophthalmol Vis Sci. 2009; 50:2710–2715.4. Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003; 110:392–399.5. Tatlpnar S, Ayata A, Unal M, Ersanl D. Fundus autofluorescence in choroidal rupture. Retin Cases Brief Rep. 2008; 2:231–233.6. Lois N, Halfyard AS, Bird AC, et al. Fundus autofluorescence in Stargardt macular dystrophy-fundus flavimaculatus. Am J Ophthalmol. 2004; 138:55–63.7. Framme C, Walter A, Gabler B, et al. Fundus autofluorescence in acute and chronic-recurrent central serous chorioretinopathy. Acta Ophthalmol Scand. 2005; 83:161–167.8. McBain VA, Townend J, Lois N. Fundus autofluorescence in exudative age-related macular degeneration. Br J Ophthalmol. 2007; 91:491–496.9. Utine CA, Tatlipinar S, Altunsoy M, Oral D, et al. Autofluorescence imaging of pingueculae. Br J Ophthalmol. 2009; 93:396–399.10. Zhang W, Shiraishi A, Suzuki A, et al. Expression and distribution of tissue transglutaminase in normal and injured rat cornea. Curr Eye Res. 2004; 28:37–45.11. Von Ruckmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995; 79:407–412.12. Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995; 36:718–729.13. Bellmann C, Holz FG, Schapp O, et al. Topography of fundus autofluorescence with a new confocal scanning laser ophthalmoscope. Ophthalmologe. 1997; 94:385–391.14. Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001; 42:1855–1866.15. Dzunic B, Jovanovic P, Veselinovic D, et al. Analysis of pathohistological characteristics of pterygium. Bosn J Basic Med Sci. 2010; 10:307–313.16. Hogan MJ, Alvarado J. Pterygium and pinguecula: electron microscopic study. Arch Ophthalmol. 1967; 78:174–186.17. Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic bases of ocular pterygia and pinguecula. Ophthalmology. 1983; 90:96–109.18. Raizada IN, Bhatnagar NK. Pinguecula and pterygium (a histopathological study). Indian J Ophthalmol. 1976; 24:16–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Patients with Pinguecula between 20 and 39 Years of Age
- Clinical Features of Pinguecula in North-Western Gyeonggi Province
- Outer Retinal Layers Alterations in Chronic Central Serous Chorioretinopathy: Spectral Domain-OCT and Fundus Autofluorescence Findings
- The Quantitative Analysis of Autofluorescence in the Aging Crystalline Lens
- The Relationship between Diabetic Retinopathy and Corneal Autofluorescence