Ann Lab Med.
2014 May;34(3):210-215. 10.3343/alm.2014.34.3.210.
Distribution of Serotypes and Antibiotic Susceptibility Patterns Among Invasive Pneumococcal Diseases in Saudi Arabia
- Affiliations
-
- 1Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia. dr.mmarieali@gmail.com
- 2Medical and Molecular Genetics Research, Clinical Laboratory Sciences Department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
- 3Department of Zoology and Biotechnology, Loyola College, Chennai, India.
- 4Department of Clinical Microbiology, Christian Medical College, Vellore, India.
- KMID: 1791922
- DOI: http://doi.org/10.3343/alm.2014.34.3.210
Abstract
- BACKGROUND
Streptococcus pneumoniae causes life-threatening infections such as meningitis, pneumonia, and febrile bacteremia, particularly in young children. The increasing number of drug-resistant isolates has highlighted the necessity for intervening and controlling disease. To achieve this, information is needed on serotype distribution and patterns of antibiotic resistance in children.
METHODS
All cases of invasive pneumococcal disease (IPD) in children aged less than 15 yr recorded at King Khalid University Hospital, King Saud University, Riyadh, Saudi Arabia, were reviewed for serotyping and antibiotic susceptibility. Isolates were collected from 78 consecutive patients with IPD between 2009 and 2012. All collected isolates were subjected to serotyping by co-agglutination, sequential multiplex PCR, and single PCR sequetyping as previously described.
RESULTS
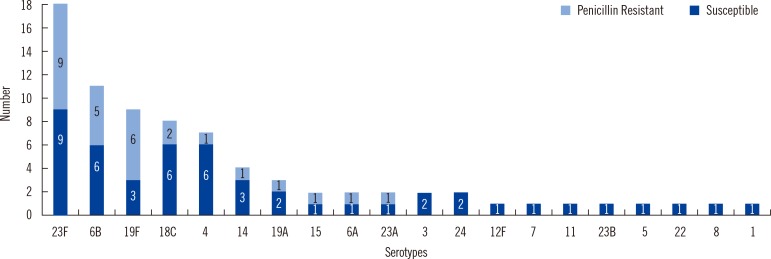
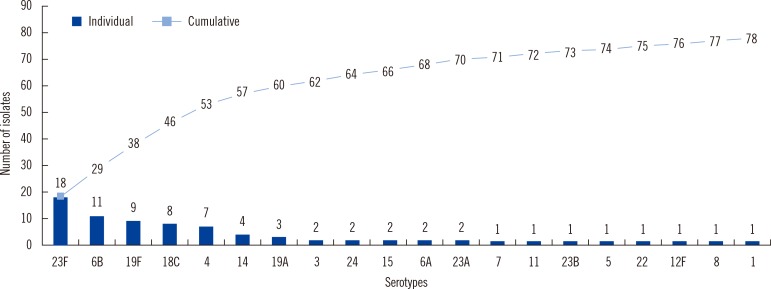
The most frequently isolated IPD serotypes were 23F, 6B, 19F, 18C, 4, 14, and 19A, which are listed in decreasing order and cover 77% of total isolates. The serotype coverage for the pneumococcal conjugate vaccine (PCV)7, PCV10, and PCV13 was 77%, 81%, and 90%, respectively. Results from sequential multiplex PCR agreed with co-agglutination results. All serotypes could not be correctly identified using single PCR sequetyping. Minimum inhibitory concentration showed that 50 (64%) isolates were susceptible to penicillin, whereas 70 (90%) isolates were susceptible to cefotaxime.
CONCLUSIONS
The most common pneumococcal serotypes occur with frequencies similar to those found in countries where the PCV has been introduced. The most common serotypes in this study are included in the PCVs. Addition of 23A and 15 to the vaccine would improve the PCV performance in IPD prevention.
MeSH Terms
-
Adolescent
Anti-Bacterial Agents/*pharmacology
Bacterial Proteins/genetics
Cefotaxime/pharmacology
Child
Child, Preschool
DNA, Bacterial/analysis
Humans
Infant
Meningitis/*diagnosis/microbiology
Microbial Sensitivity Tests
Multiplex Polymerase Chain Reaction
Penicillins/pharmacology
Pneumococcal Vaccines/immunology
Pneumonia/*diagnosis/microbiology
Protein Tyrosine Phosphatases/genetics
Retrospective Studies
Saudi Arabia
Serotyping
Streptococcus pneumoniae/*drug effects/genetics
Anti-Bacterial Agents
Bacterial Proteins
Cefotaxime
DNA, Bacterial
Penicillins
Pneumococcal Vaccines
Protein Tyrosine Phosphatases
Figure
Reference
-
1. Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. Accuracy of phenotypic methods for identification of Streptococcus pneumoniae isolates included in surveillance programs. J Clin Microbiol. 2008; 46:2184–2188. PMID: 18495854.2. Arbique JC, Poyart C, Trieu-Cuot P, Quesne G, Carvalho Mda G, Steigerwalt AG, et al. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J Clin Microbiol. 2004; 42:4686–4696. PMID: 15472328.3. James J, Gnanapragasam HP, Sangeetha G. Causes and epidemiology of vaccine preventable infectious bacterial disease: The prospect and short out coming of vaccine. Int J Pharm Pharm Sci. 2012; 4:51–54.4. Dias CA, Teixeira LM, Carvalho Mda G, Beall B. Sequential multiplex PCR for determining capsular serotypes of pneumococci recovered from Brazilian children. J Med Microbiol. 2007; 56:1185–1188. PMID: 17761481.
Article5. Lee JH, Kim SH, Lee J, Choi EH, Lee HJ. Diagnosis of pneumococcal empyema using immunochromatographic test on pleural fluid and serotype distribution in Korean children. Diagn Microbiol Infect Dis. 2012; 72:119–124. PMID: 22079140.
Article6. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000; 30:100–121. PMID: 10619740.
Article7. Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007; 75:4482–4489. PMID: 17576753.
Article8. Pimenta FC, Roundtree A, Soysal A, Bakir M, du Plessis M, Wolter N, et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. J Clin Microbiol. 2013; 51:647–652. PMID: 23224094.
Article9. Paradiso PR. Advances in pneumococcal disease prevention: 13-valent pneumococcal conjugate vaccine for infants and children. Clin Infect Dis. 2011; 52:1241–1247. PMID: 21507921.
Article10. Fedson DS, Guppy MJ. Pneumococcal vaccination of older adults: conjugate or polysaccharide? Hum Vaccin Immunother. 2013; 9:1382–1384. PMID: 23732892.11. Brito DA, Ramirez M, de Lencastre H. Serotyping Streptococcus pneumoniae by multiplex PCR. J Clin Microbiol. 2003; 41:2378–2384. PMID: 12791852.12. Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006; 44:124–131. PMID: 16390959.
Article13. Leung MH, Bryson K, Freystatter K, Pichon B, Edwards G, Charalambous BM, et al. Sequetyping: serotyping Streptococcus pneumoniae by a single PCR sequencing strategy. J Clin Microbiol. 2012; 50:2419–2427. PMID: 22553238.14. Greve T, Møller JK. Accuracy of using the lytA gene to distinguish Streptococcus pneumoniae from related species. J Med Microbiol. 2012; 61:478–482. PMID: 22135022.15. Lalitha MK, Thomas K, Kumar RS, Steinhoff MC. the IBIS Study Group. Serotyping of Streptococcus pneumoniae by coagglutination with 12 pooled antisera. J Clin Microbiol. 1999; 37:263–265. PMID: 9854110.16. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 29th Informational supplement, M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute;2009.17. Shibl AM, Memish ZA, Al-Kattan KM. Antibiotic resistance and serotype distribution of invasive pneumococcal diseases before and after introduction of pneumococcal conjugate vaccine in the Kingdom of Saudi Arabia (KSA). Vaccine. 2012; 30(S6):G32–G36. PMID: 23228355.
Article18. Dagan R. Impact of pneumococcal conjugate vaccine on infections caused by antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Infect. 2009; 15(S3):16–20. PMID: 19366365.19. Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007; 196:1346–1354. PMID: 17922399.
Article20. Al-Mazrou A, Twum-Danso K, Al Zamil F, Kambal A. Streptococcus pneumoniae serotypes/serogroups causing invasive disease in Riyadh, Saudi Arabia: extent of coverage by pneumococcal vaccines. Ann Saudi Med. 2005; 25:94–99. PMID: 15977684.21. Fouda SI, Kadry AA, Shibl AM. Beta-lactam and macrolide resistance and serotype distribution among Streptococcus pneumoniae isolates from Saudi Arabia. J Chemother. 2004; 16:517–523. PMID: 15700841.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Group B Streptococcus Colonization, Antibiotic Susceptibility, and Serotype Distribution among Saudi Pregnant Women
- Direct and Indirect Effects of Pneumococcal Protein Conjugate Vaccine
- Serotype Distribution and Antimicrobial Susceptibilities of Invasive Streptococcus pneumoniae Isolates from Adults in Korea from 1997 to 2012
- Efficacy and effectiveness of pneumococcal conjugate vaccine in children
- Recommendation for use of the newly introduced pneumococcal protein conjugate vaccines in Korea