Improvement Characteristics of Bio-active Materials Coated Fabric on Rat Muscular Mitochondria
- Affiliations
-
- 1Department of Physiology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea. akdongyi01@cau.ac.kr
- 2Department of Family Medicine, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 3Research and Development Center, VENTEX Co. Ltd., Seoul 138-220, Korea.
- KMID: 1791431
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.283
Abstract
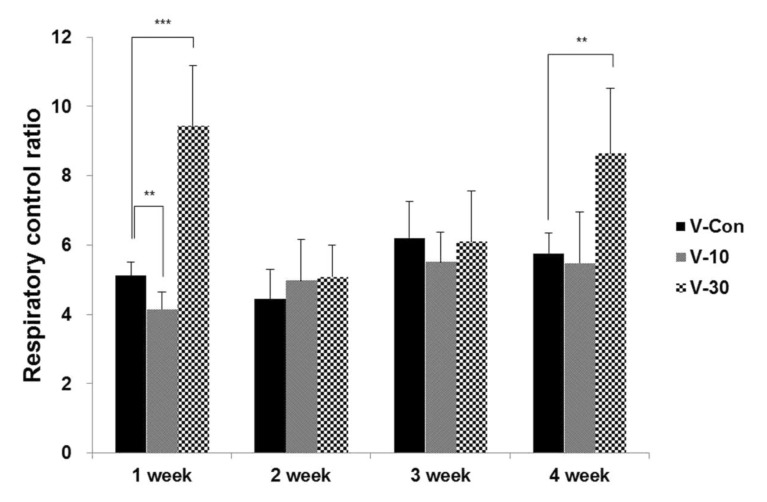
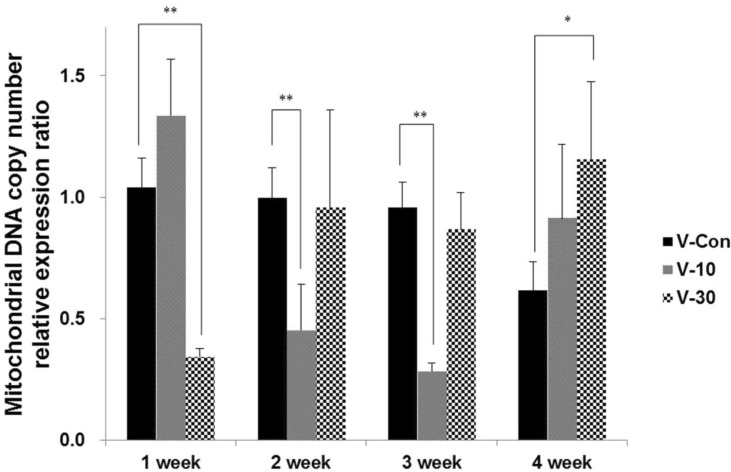
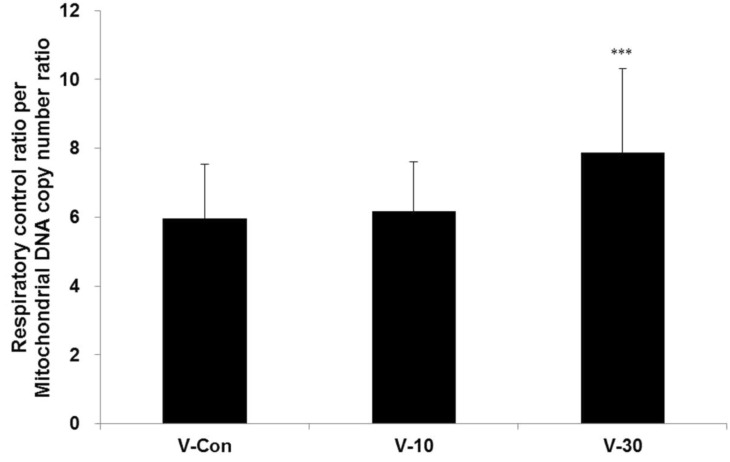
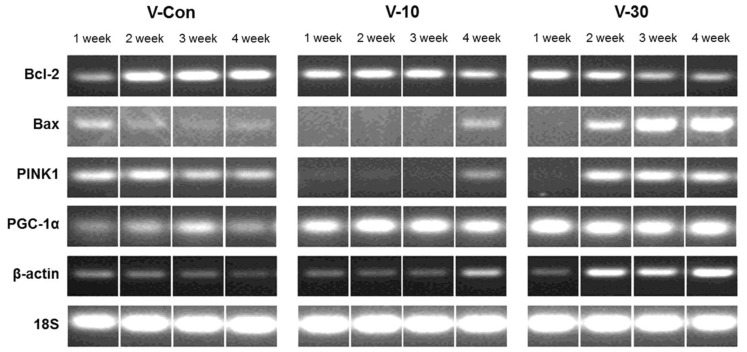
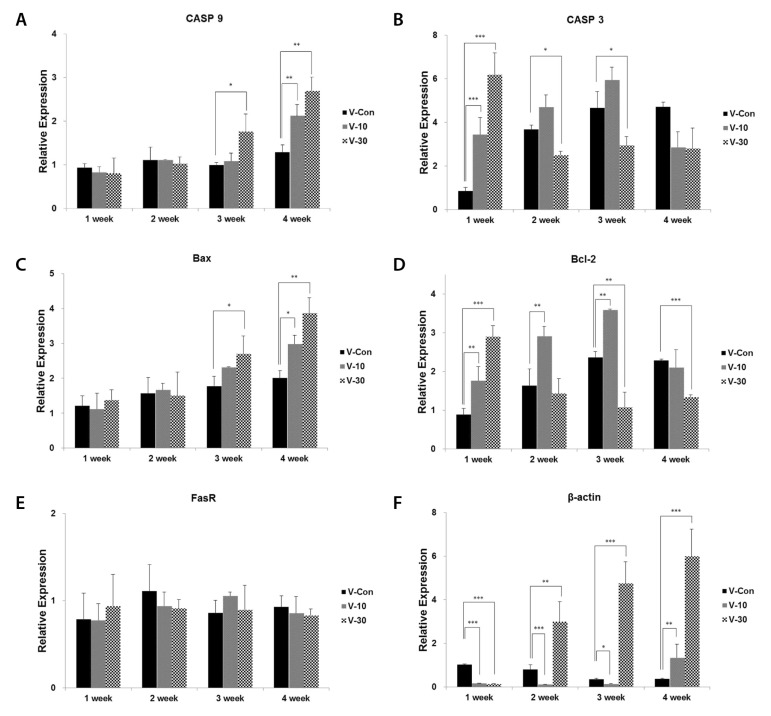
- This study surveys the improvement characteristics in old-aged muscular mitochondria by bio-active materials coated fabric (BMCF). To observe the effects, the fabric (10 and 30%) was worn to old-aged rat then the oxygen consumption efficiency and copy numbers of mitochondria, and mRNA expression of apoptosis- and mitophagy-related genes were verified. By wearing the BMCF, the oxidative respiration significantly increased when using the 30% materials coated fabric. The mitochondrial DNA copy number significantly decreased and subsequently recovered in a dose-dependent manner. The respiratory control ratio to mitochondrial DNA copy number showed a dose-dependent increment. As times passed, Bax, caspase 9, PGC-1alpha and beta-actin increased, and Bcl-2 decreased in a dose-dependent manner. However, the BMCF can be seen to have had no effect on Fas receptor. PINK1 expression did not change considerably and was inclined to decrease in control group, but the expression was down-regulated then subsequently increased with the use of the BMCF in a dose-dependent manner. Caspase 3 increased and subsequently decreased in a dose-dependent manner. These results suggest that the BMCF invigorates mitophagy and improves mitochondrial oxidative respiration in skeletal muscle, and in early stage of apoptosis induced by the BMCF is not related to extrinsic death-receptor mediated but mitochondria-mediated signaling pathway.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling
Donghee Lee, Yelim Seo, Young-Won Kim, Seongtae Kim, Hyemi Bae, Jeongyoon Choi, Inja Lim, Hyoweon Bang, Jung-Ha Kim, Jae-Hong Ko
Korean J Physiol Pharmacol. 2019;23(2):141-150. doi: 10.4196/kjpp.2019.23.2.141.Far-infrared rays enhance mitochondrial biogenesis and
GLUT3 expression under low glucose conditions in rat skeletal muscle cells
Yelim Seo, Young-Won Kim, Donghee Lee, Donghyeon Kim, Kyoungseo Kim, Taewoo Kim, Changyeob Baek, Yerim Lee, Junhyeok Lee, Hosung Lee, Geonwoo Jang, Wonyeong Jeong, Junho Choi, Doegeun Hwang, Jung Soo Suh, Sun-Woo Kim, Hyoung Kyu Kim, Jin Han, Hyoweon Bang, Jung-Ha Kim, Tong Zhou, Jae-Hong Ko
Korean J Physiol Pharmacol. 2021;25(2):167-175. doi: 10.4196/kjpp.2021.25.2.167.
Reference
-
1. Ibrahim NA, Eid BM, Khalil HM. Cellulosic/wool pigment prints with remarkable antibacterial functionalities. Carbohydr Polym. 2015; 115:559–567. PMID: 25439932.
Article2. Fluhr JW, Breternitz M, Kowatzki D, Bauer A, Bossert J, Elsner P, Hipler UC. Silver-loaded seaweed-based cellulosic fiber improves epidermal skin physiology in atopic dermatitis: safety assessment, mode of action and controlled, randomized single-blinded exploratory in vivo study. Exp Dermatol. 2010; 19:e9–e15. PMID: 19645851.
Article3. Koller DY, Halmerbauer G, Böck A, Engstler G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: a 3-month trial. Pediatr Allergy Immunol. 2007; 18:335–338. PMID: 17346297.4. Ricci G, Patrizi A, Bendandi B, Menna G, Varotti E, Masi M. Clinical effectiveness of a silk fabric in the treatment of atopic dermatitis. Br J Dermatol. 2004; 150:127–131. PMID: 14746626.
Article5. Lee MS, Song J, Kim HJ, Park KW, Moon SR. Effect of multi-functional fabric on sleep stages and growth hormone levels during sleep. Int J Neurosci. 2004; 114:795–804. PMID: 15204045.
Article6. Lee MS, Kim HJ, Song J, Park KW, Moon SR. Effects of multifunctional fabrics on cardiac autonomic tone and psychological state. Int J Neurosci. 2004; 114:923–931. PMID: 15527199.7. Herbst A, Johnson CJ, Hynes K, McKenzie D, Aiken JM. Mitochondrial biogenesis drives a vicious cycle of metabolic insufficiency and mitochondrial DNA deletion mutation accumulation in aged rat skeletal muscle fibers. PLoS One. 2013; 8:e59006. PMID: 23516592.
Article8. Wagatsuma A, Kotake N, Yamada S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol Cell Biochem. 2011; 349:139–147. PMID: 21110070.
Article9. Remels AH, Langen RC, Schrauwen P, Schaart G, Schols AM, Gosker HR. Regulation of mitochondrial biogenesis during myogenesis. Mol Cell Endocrinol. 2010; 315:113–120. PMID: 19804813.
Article10. Duguez S, Féasson L, Denis C, Freyssenet D. Mitochondrial biogenesis during skeletal muscle regeneration. Am J Physiol Endocrinol Metab. 2002; 282:E802–E809. PMID: 11882500.11. Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, Wrutniak C, Cabello G. Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J Biol Chem. 2000; 275:2733–2744. PMID: 10644737.
Article12. Barbieri E, Battistelli M, Casadei L, Vallorani L, Piccoli G, Guescini M, Gioacchini AM, Polidori E, Zeppa S, Ceccaroli P, Stocchi L, Stocchi V, Falcieri E. Morphofunctional and biochemical approaches for studying mitochondrial changes during myoblasts differentiation. J Aging Res. 2011; 2011:845379. PMID: 21629710.
Article13. Seyer P, Grandemange S, Rochard P, Busson M, Pessemesse L, Casas F, Cabello G, Wrutniak-Cabello C. P43-dependent mitochondrial activity regulates myoblast differentiation and slow myosin isoform expression by control of Calcineurin expression. Exp Cell Res. 2011; 317:2059–2071. PMID: 21664352.
Article14. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006; 16:R551–R560. PMID: 16860735.
Article15. McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease--its impact, etiology, and pathology. Curr Top Dev Biol. 2007; 77:113–155. PMID: 17222702.
Article16. Giorgi C, Wieckowski MR, Pandolfi PP, Pinton P. Mitochondria associated membranes (MAMs) as critical hubs for apoptosis. Commun Integr Biol. 2011; 4:334–335. PMID: 21980573.
Article17. Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999; 341:233–249. PMID: 10393078.
Article18. Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010; 40:280–293. PMID: 20965422.
Article19. Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010; 12:842–846. PMID: 20811357.
Article20. Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal. 2010; 12:503–535. PMID: 19650712.
Article21. Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006; 209:2265–2275. PMID: 16731803.
Article22. Hock MB, Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol. 2009; 71:177–203. PMID: 19575678.
Article23. Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007; 462:245–253. PMID: 17475204.
Article24. Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007; 2:287–295. PMID: 17406588.25. Guo W, Jiang L, Bhasin S, Khan SM, Swerdlow RH. DNA extraction procedures meaningfully influence qPCR-based mtDNA copy number determination. Mitochondrion. 2009; 9:261–265. PMID: 19324101.
Article26. Korohoda W, Pietrzkowski Z, Reiss K. Chloramphenicol, an inhibitor of mitochondrial protein synthesis, inhibits myoblast fusion and myotube differentiation. Folia Histochem Cytobiol. 1993; 31:9–13. PMID: 8500631.27. Herzberg NH, Middelkoop E, Adorf M, Dekker HL, Van Galen MJ, Van den Berg M, Bolhuis PA, Van den Bogert C. Mitochondria in cultured human muscle cells depleted of mitochondrial DNA. Eur J Cell Biol. 1993; 61:400–408. PMID: 8223726.28. Hamai N, Nakamura M, Asano A. Inhibition of mitochondrial protein synthesis impaired C2C12 myoblast differentiation. Cell Struct Funct. 1997; 22:421–431. PMID: 9368716.
Article29. Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994; 124:1–6. PMID: 8294493.
Article30. Parker JE, Mufti GJ, Rasool F, Mijovic A, Devereux S, Pagliuca A. The role of apoptosis, proliferation, and the Bcl-2-related proteins in the myelodysplastic syndromes and acute myeloid leukemia secondary to MDS. Blood. 2000; 96:3932–3938. PMID: 11090080.
Article31. Zhang XD, Wang Y, Wu JC, Lin F, Han R, Han F, Fukunaga K, Qin ZH. Down-regulation of Bcl-2 enhances autophagy activation and cell death induced by mitochondrial dysfunction in rat striatum. J Neurosci Res. 2009; 87:3600–3610. PMID: 19565656.
Article32. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006; 27:728–735. PMID: 17018837.33. Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004; 18:357–368. PMID: 15004004.
Article34. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999; 98:115–124. PMID: 10412986.
Article35. Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012; 125:795–799. PMID: 22448035.
Article36. Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010; 189:211–221. PMID: 20404107.
Article37. Clarke P, Tyler KL. Apoptosis in animal models of virus-induced disease. Nat Rev Microbiol. 2009; 7:144–155. PMID: 19148180.
Article38. Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat Rev Neurosci. 2008; 9:344–356. PMID: 18425089.
Article39. Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998; 1366:177–196. PMID: 9714796.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myopathy, Drugs, and Mitochondria
- Improvement effects of electrochemical stability of magnetic materials for prosthetic dentistry
- Biochemical and Physiological Characteristics of Ca-ATPase System of Rat Liver Mitochondria with Special Attention to the Effects of pH and Temperature
- Fine Structure Alteration of Rat Liver induced by Nitrosohexamethylenamine
- CLINICAL STUDY OF ENDOSSEOUS HYDROXYAPATITE COATED IMPLANTS