J Korean Med Sci.
2013 Apr;28(4):534-541. 10.3346/jkms.2013.28.4.534.
Correlation of Immunohistochemical Markers and BRAF Mutation Status with Histological Variants of Papillary Thyroid Carcinoma in the Korean Population
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. lilloa@snuh.org
- KMID: 1786960
- DOI: http://doi.org/10.3346/jkms.2013.28.4.534
Abstract
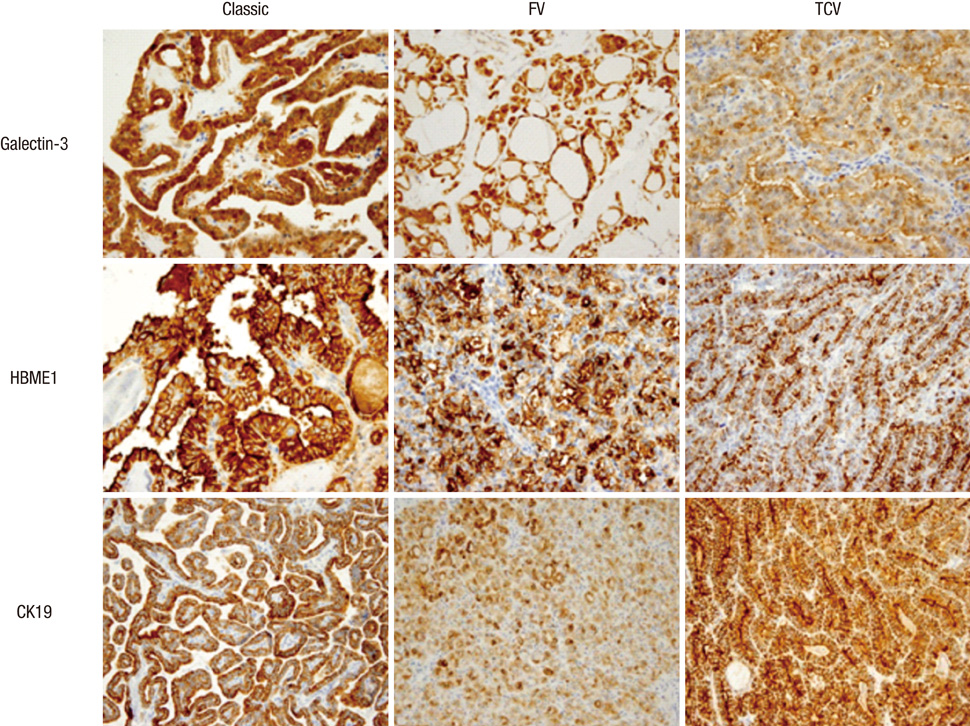
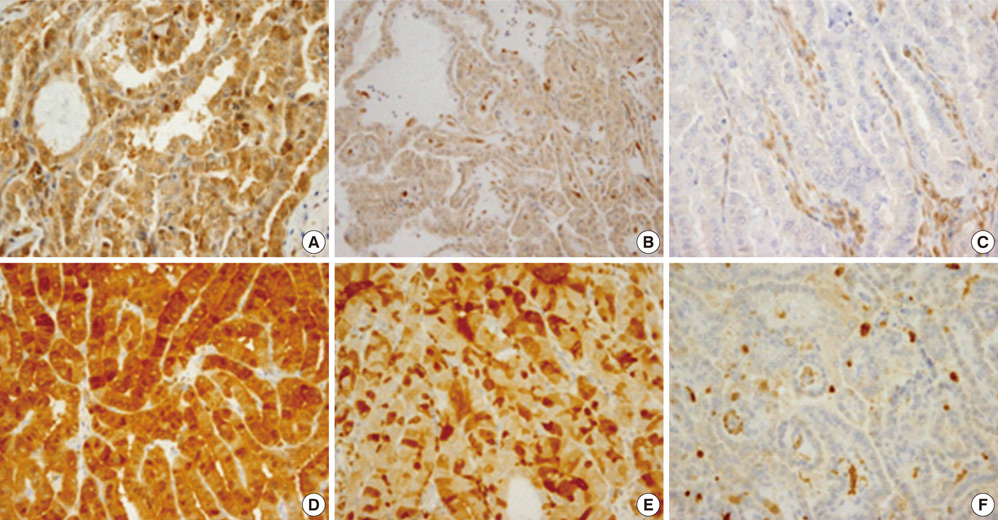
- Several pathologic characteristics are associated with an adverse clinical outcome in papillary thyroid carcinoma (PTC), including the histological variant. This study aimed to investigate immunohistochemical expression and BRAF mutation status based on the histological variant and evaluated potential markers of aggressive behavior of PTC in Korean patients. In all, 407 PTC cases were classified to each histological variant, and the 94 representative cases were subjected to immunohistochemistry and BRAF mutation analysis. The classic type, follicular variant (FV) and tall cell variant (TCV) represented 76.9%, 14.2% and 6%, respectively. TCV showed a larger tumor size (P = 0.009), frequent extrathyroidal extension (P = 0.022) and cervical lymph node (LN) metastasis (P = 0.018). TCV and FV showed the reduced expression of galectin-3 (P = 0.003) and HBME1 (P = 0.114). Regardless of histology, PTEN loss and diffuse S100A4 expression were associated with LN metastasis (P = 0.007, P = 0.013). All TCVs harbored BRAF V600E mutation, and FV harbored less BRAF V600E mutation (P = 0.043). Immunohistochemical evaluation showed characteristic patterns in histological variants. PTEN and S100A4 expression are suggested as indicators of regional lymph node metastasis.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Asian Continental Ancestry Group/*genetics
Carcinoma, Papillary/genetics/metabolism/*pathology
DNA Mutational Analysis
Exons
Female
Galectin 3/metabolism
Humans
Immunohistochemistry
Lymphatic Metastasis
Male
Middle Aged
Mutation
PTEN Phosphohydrolase/metabolism
Proto-Oncogene Proteins B-raf/*genetics/metabolism
Republic of Korea
S100 Proteins/metabolism
Thyroid Neoplasms/genetics/metabolism/*pathology
Tumor Markers, Biological/metabolism
Young Adult
Galectin 3
S100 Proteins
Tumor Markers, Biological
Proto-Oncogene Proteins B-raf
PTEN Phosphohydrolase
Figure
Reference
-
1. Akslen LA, LiVolsi VA. Prognostic significance of histologic grading compared with subclassification of papillary thyroid carcinoma. Cancer. 2000. 88:1902–1908.2. Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, Kihara M, Miyauchi A. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. 2008. 99:1908–1915.3. Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid. 2010. 20:153–158.4. LiVolsi VA. Papillary carcinoma tall cell variant (TCV): a review. Endocr Pathol. 2010. 21:12–15.5. Ghossein R, Livolsi VA. Papillary thyroid carcinoma tall cell variant. Thyroid. 2008. 18:1179–1181.6. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001. 86:656–658.7. Zou M, Al-Baradie RS, Al-Hindi H, Farid NR, Shi Y. S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. Br J Cancer. 2005. 93:1277–1284.8. Min HS, Choe G, Kim SW, Park YJ, Park do J, Youn YK, Park SH, Cho BY, Park SY. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod Pathol. 2008. 21:748–755.9. Murakawa T, Tsuda H, Tanimoto T, Tanabe T, Kitahara S, Matsubara O. Expression of KIT, EGFR, HER-2 and tyrosine phosphorylation in undifferentiated thyroid carcinoma: implication for a new therapeutic approach. Pathol Int. 2005. 55:757–765.10. Landriscina M, Pannone G, Piscazzi A, Toti P, Fabiano A, Tortorella S, Occhini R, Ambrosi A, Bufo P, Cignarelli M. Epidermal growth factor receptor 1 expression is upregulated in undifferentiated thyroid carcinomas in humans. Thyroid. 2011. 21:1227–1234.11. Freudenberg LS, Sheu S, Görges R, Mann K, Bokler S, Frilling A, Schmid KW, Bockisch A, Otterbach F. Prognostic value of c-erbB-2 expression in papillary thyroid carcinoma. Nuklearmedizin. 2005. 44:179–182. 18412. Wang Y, Hou P, Yu H, Wang W, Ji M, Zhao S, Yan S, Sun X, Liu D, Shi B, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab. 2007. 92:2387–2390.13. Alvarez-Nuñez F, Bussaglia E, Mauricio D, Ybarra J, Vilar M, Lerma E, de Leiva A, Matias-Guiu X. Thyroid Neoplasia Study Group. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006. 16:17–23.14. Sozopoulos E, Litsiou H, Voutsinas G, Mitsiades N, Anagnostakis N, Tseva T, Patsouris E, Tseleni-Balafouta S. Mutational and immunohistochemical study of the PI3K/Akt pathway in papillary thyroid carcinoma in Greece. Endocr Pathol. 2010. 21:90–100.15. Ito Y, Hirokawa M, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Prevalence and biological behaviour of variants of papillary thyroid carcinoma: experience at a single institute. Pathology. 2008. 40:617–622.16. Ito Y, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, Matsuzuka F, Kakudo K, Kuma K, Miyauchi A. S100A4 expression is an early event of papillary carcinoma of the thyroid. Oncology. 2004. 67:397–402.17. De Araujo-Filho VJ, Alves VA, de Castro IV, Lourenço SV, Cernea CR, Brandão LG, Ferraz AR. Vascular endothelial growth factor expression in invasive papillary thyroid carcinoma. Thyroid. 2009. 19:1233–1237.18. Prasad ML, Pellegata NS, Huang Y, Nagaraja HN, de la Chapelle A, Kloos RT. Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol. 2005. 18:48–57.19. Barut F, Onak Kandemir N, Bektas S, Bahadir B, Keser S, Ozdamar SO. Universal markers of thyroid malignancies: galectin-3, HBME-1, and cytokeratin-19. Endocr Pathol. 2010. 21:80–89.20. Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular-derived thyroid nodules. Diagn Pathol. 2010. 5:9.21. Jung CK, Choi YJ, Lee KY, Bae JS, Kim HJ, Yoon SK, Son YI, Chung JH, Oh YL. The cytological, clinical, and pathological features of the cribriform-morular variant of papillary thyroid carcinoma and mutation analysis of CTNNB1 and BRAF genes. Thyroid. 2009. 19:905–913.22. Khoo ML, Beasley NJ, Ezzat S, Freeman JL, Asa SL. Overexpression of cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002. 87:1814–1818.23. Westermark K, Lundqvist M, Wallin G, Dahlman T, Hacker GW, Heldin NE, Grimelius L. EGF-receptors in human normal and pathological thyroid tissue. Histopathology. 1996. 28:221–227.24. Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol. 2000. 156:1693–1700.25. Hollander MC, Blumenthal GM, Dennis PA. PTEN loss in the continuum of common cancers, rare syndromes and mouse models. Nat Rev Cancer. 2011. 11:289–301.26. Jung CK, Im SY, Kang YJ, Lee H, Jung ES, Kang CS, Bae JS, Choi YJ. Mutational patterns and novel mutations of the BRAF gene in a large cohort of Korean patients with papillary thyroid carcinoma. Thyroid. 2012. 22:791–797.27. Kim KH, Suh KS, Kang DW, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma and in Hashimoto’s thyroiditis. Pathol Int. 2005. 55:540–545.28. Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, Qumsiyeh MB, Rothstein JL, Fusco A, Santoro M, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006. 91:2414–2423.29. Bhaijee F, Nikiforov YE. Molecular analysis of thyroid tumors. Endocr Pathol. 2011. 22:126–133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multifocal Papillary Thyroid Carcinoma Consisting of One Encapsulated Follicular Variant with BRAF K601E Mutation and Three Conventional Types with BRAF V600E Mutation
- Relation between RASSF1A Methylation and BRAF Mutation in Thyroid Tumor
- Medullary and Papillary Thyroid Carcinoma as a Collision Tumor: Report of Five Cases
- Clinical Implication of BRAF Mutation in Thyroid Cancer
- Association of BRAF(V600E) Mutation with Poor Clinical Prognostic Factors and Ultrasonographic Findings in Cases of Papillary Thyroid Carcinoma