J Korean Med Sci.
2013 Apr;28(4):527-533. 10.3346/jkms.2013.28.4.527.
The Synergistic Apoptotic Interaction of Indole-3-Carbinol and Genistein with TRAIL on Endometrial Cancer Cells
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Keimyung University, School of Medicine, Daegu, Korea. c0035@dsmc.or.kr
- 2Institute for Cancer Research, Keimyung University, School of Medicine, Daegu, Korea.
- 3Department of Oncology, Lombardi Cancer Center, Georgetown University, Washington DC, USA.
- KMID: 1786959
- DOI: http://doi.org/10.3346/jkms.2013.28.4.527
Abstract
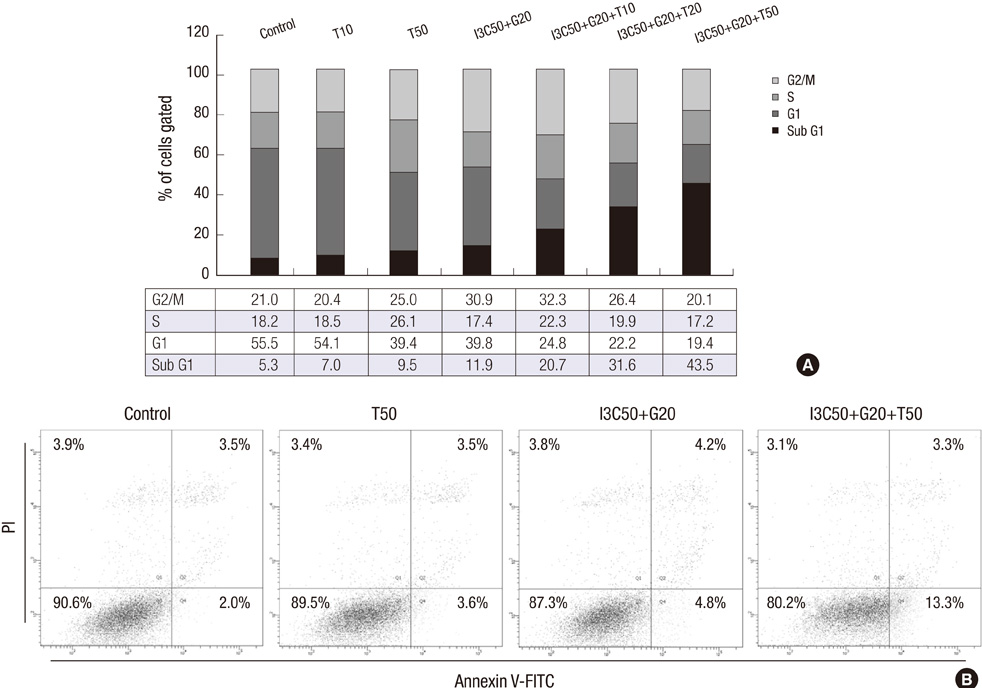
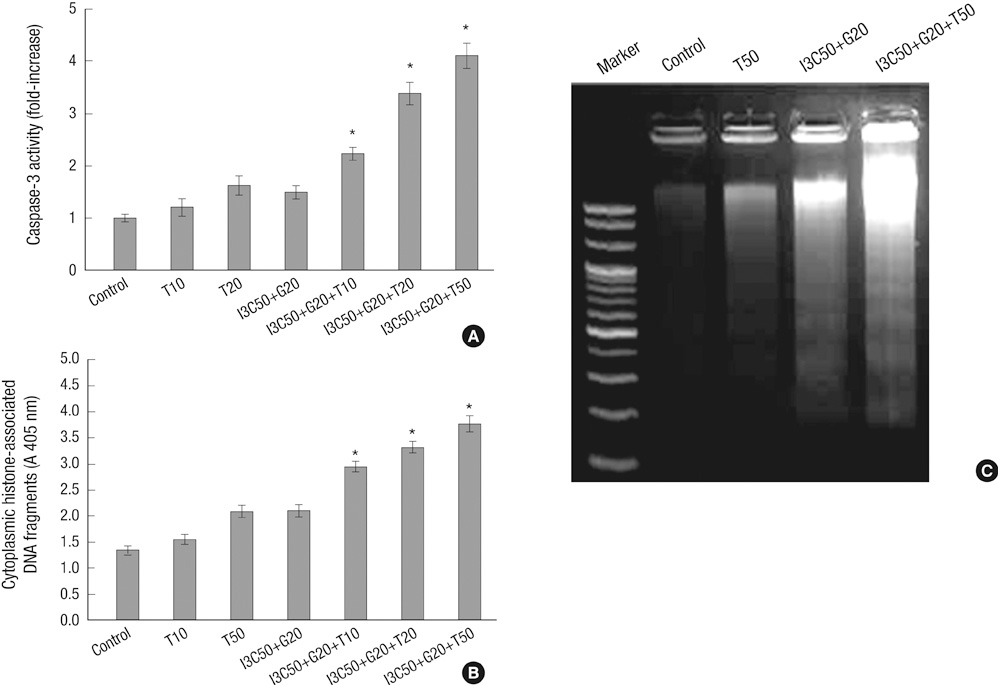
- Induction of apoptosis in target cells is a key mechanism by which chemotherapy promotes cell killing. The purpose of this study was to determine whether Indole-3-Carbinol (I3C) and Genistein in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induce apoptosis in endometrial cancer cell (Ishikawa) and to assess apoptotic mechanism. The MTT assay and flow cytometry were performed to determine cell viability and cell cycle. The induction of apoptosis was measured by caspase-3 activity test, DNA fragmentation assay, annexin V binding assay and western blot analysis. There was no effect in cell growth inhibition and cell cycle progression alone or in two-combination. However, the treatment of I3C and Genistein followed by TRAIL showed significant cell death and marked increase in sub-G1 arrest. Three-combination treatment revealed elevated expression of DR4, DR5 and cleaved forms of caspase-3, caspase-8, PARP. The Flip was found down regulated. Moreover, increase in caspase-3 activity and DNA fragmentation indicated the induction of apoptosis. The results indicate that I3C and Genistein with TRAIL synergistically induced apoptosis via death receptor dependent pathway. Our findings might provide a new insight into the development of novel combination therapies against endometrial cancer.
Keyword
MeSH Terms
-
Anticarcinogenic Agents/*pharmacology
Apoptosis/*drug effects
Caspase 3/metabolism
Caspase 8/metabolism
Cell Line, Tumor
Drug Synergism
Endometrial Neoplasms/metabolism/pathology
Female
G1 Phase Cell Cycle Checkpoints/drug effects
Genistein/*pharmacology
Humans
Indoles/*pharmacology
Poly(ADP-ribose) Polymerases/metabolism
Receptors, TNF-Related Apoptosis-Inducing Ligand/metabolism
TNF-Related Apoptosis-Inducing Ligand/*pharmacology
Anticarcinogenic Agents
Indoles
Receptors, TNF-Related Apoptosis-Inducing Ligand
TNF-Related Apoptosis-Inducing Ligand
Genistein
Poly(ADP-ribose) Polymerases
Caspase 3
Caspase 8
Figure
Reference
-
1. Gayther SA, Pharoah PD. The inherited genetics of ovarian and endometrial cancer. Curr Opin Genet Dev. 2010. 20:231–238.2. Homesley HD. Present status and future direction of clinical trials in advanced endometrial carcinoma. J Gynecol Oncol. 2008. 19:157–161.3. Frantz S. Drug discovery: playing dirty. Nature. 2005. 437:942–943.4. Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006. 17:659–664.5. Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008. 269:226–242.6. Oki T, Sowa Y, Hirose T, Takagaki N, Horinaka M, Nakanishi R, Yasuda C, Yoshida T, Kanazawa M, Satomi Y, et al. Genistein induces Gadd45 gene and G2/M cell cycle arrest in the DU145 human prostate cancer cell line. FEBS Lett. 2004. 577:55–59.7. Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12]Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988. 157:183–189.8. Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999. 104:155–162.9. Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003. 22:8628–8633.10. Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001. 299:31–38.11. Nakamura Y, Yogosawa S, Izutani Y, Watanabe H, Otsuji E, Sakai T. A combination of indol-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol Cancer. 2009. 8:100.12. Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int. 2009. 33:1237–1244.13. Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007. 80:1403–1408.14. LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003. 10:66–75.15. Dolcet X, Llobet D, Pallares J, Rue M, Comella JX, Matias-Guiu X. FLIP is frequently expressed in endometrial carcinoma and has a role in resistance to TRAIL-induced apoptosis. Lab Invest. 2005. 85:885–894.16. Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia. 2001. 3:535–546.17. Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997. 388:190–195.18. Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002. 277:25020–25025.19. Abedini MR, Qiu Q, Yan X, Tsang BK. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004. 23:6997–7004.20. Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008. 8:37–46.21. Tomek S, Horak P, Pribill I, Haller G, Rössler M, Zielinski CC, Pils D, Krainer M. Resistance to TRAIL-induced apoptosis in ovarian cancer cell lines is overcome by co-treatment with cytotoxic drugs. Gynecol Oncol. 2004. 94:107–114.22. Lane D, Cartier A, L'Espérance S, Côté M, Rancourt C, Piché A. Differential induction of apoptosis by tumor necrosis factor-related apoptosis-inducing ligand in human ovarian carcinoma cells. Gynecol Oncol. 2004. 93:594–604.23. Weng JR, Tsai CH, Kulp SK, Wang D, Lin CH, Yang HC, Ma Y, Sargeant A, Chiu CF, Tsai MH, et al. A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007. 67:7815–7824.24. Jeon KI, Rih JK, Kim HJ, Lee YJ, Cho CH, Goldberg ID, Rosen EM, Bae I. Pretreatment of indole-3-carbinol augments TRAIL-induced apoptosis in a prostate cancer cell line, LNCaP. FEBS Lett. 2003. 544:246–251.25. Jin CY, Park C, Cheong J, Choi BT, Lee TH, Lee JD, Lee WH, Kim GY, Ryu CH, Choi YH. Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett. 2007. 257:56–64.26. Szliszka E, Krol W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol Rep. 2011. 26:533–541.27. Jin CY, Park C, Moon SK, Kim GY, Kwon TK, Lee SJ, Kim WJ, Choi YH. Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs. 2009. 20:713–722.28. Szliszka E, Czuba ZP, Jernas K, Król W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int J Mol Sci. 2008. 9:56–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indole-3-carbinol and genistein inhibit growth of human uterine leiomyoma cells
- The Activation of ERK1/2 Via Tyrosine Kinase Pathway Attenuates TRAIL-induced Apoptosis in HeLa cell
- Effect of Indole-3-Carbinol on Inhibition of MMP Activity via MAPK Signaling Pathway in Human Prostate Cancer Cell Line, PC3 Cells
- Troglitazone Increases the Susceptibility to TRAIL-Induced Apoptosis in Thyroid Cancer Cell Lines
- Parthenolide Sensitizes Human Colorectal Cancer Cells to Tumor Necrosis Factor-related Apoptosis-inducing Ligand through Mitochondrial and Caspase Dependent Pathway