J Korean Med Sci.
2004 Jun;19(3):419-425. 10.3346/jkms.2004.19.3.419.
Upregulation of Glutamate Receptors in Rat Cerebral Cortex with Neuronal Migration Disorders
- Affiliations
-
- 1Department of Pathology, Chonnam National University Medical School, Gwangju, Korea. mclee@jnu.ac.kr
- 2Epilepsy Clinic and Research Group, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Neurosurgery, Veterans Hospital, Gwangju, Korea.
- KMID: 1786822
- DOI: http://doi.org/10.3346/jkms.2004.19.3.419
Abstract
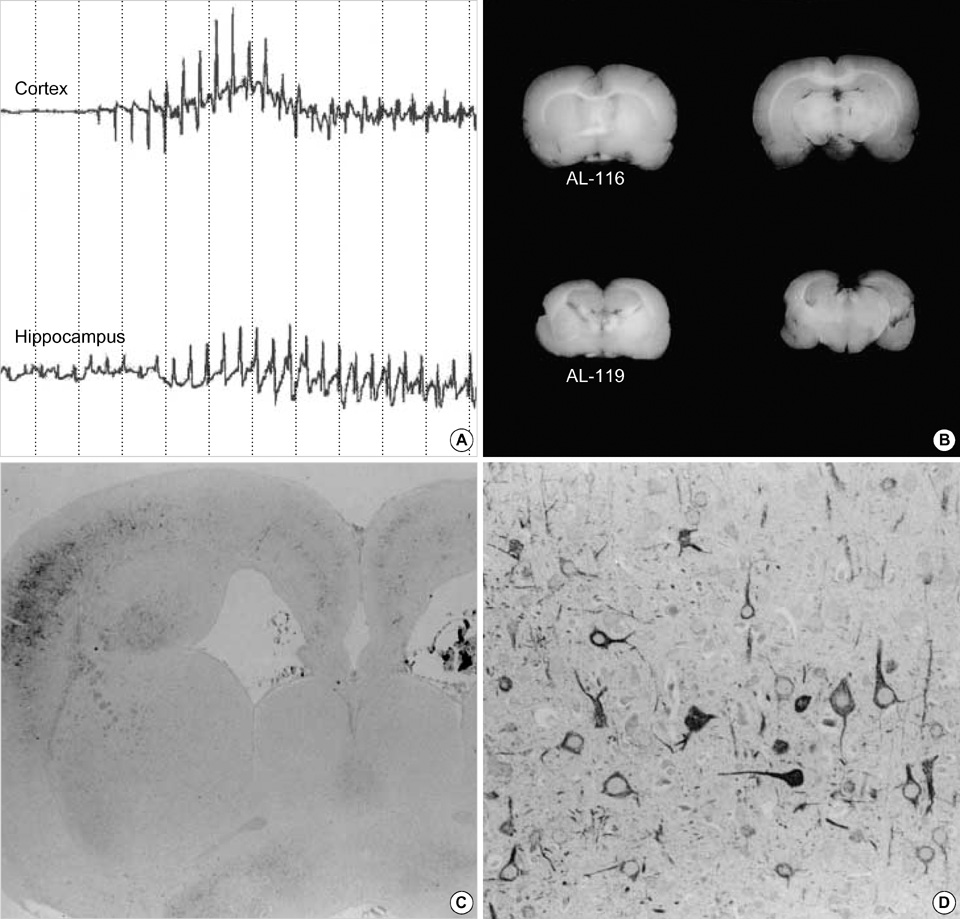
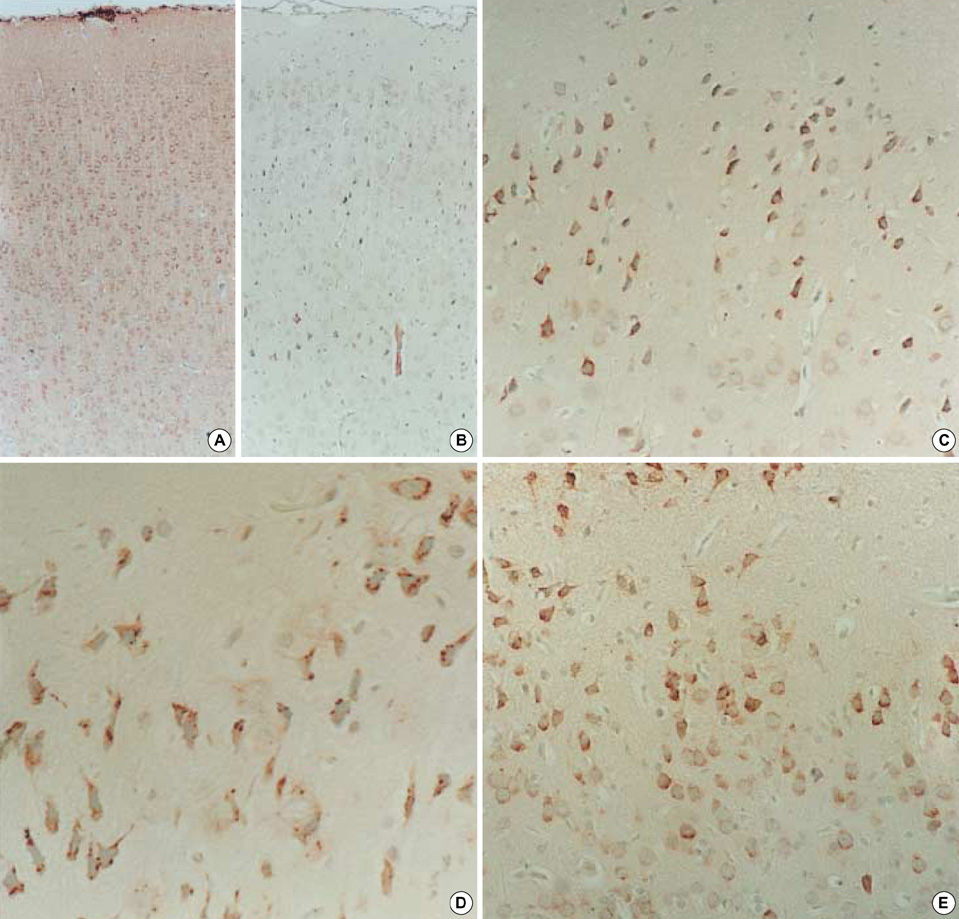
- Neuronal migration disorders (NMDs) constitute the main pathologic substrate of medically intractable epilepsy in human. This study is designed to investigate the changes in expression of glutamate receptor subtypes on radiation-induced NMD in rats. The lesion was produced by intrauterine irradiation (240 cGy) on E17 rats, and then 10 weeks old rats were used for the study. The pathologic and immunohistochemical findings for glutamate receptor subunit proteins on NMD cortex were correlated with development of behavioral seizures and EEG abnormality. Spontaneous seizures uncommonly occurred in NMD rats (5%); however, clinical stages of seizures were significantly increased in NMD rats by an administration of kainic acid. Brains taken from irradiated rats revealed gross and histopathologic features of NMD. Focal cortical dysplasia was identified by histopathology and immunohistochemistry with neurofilament protein (NF-M/H). Significantly strong NR1 and NR2A/B immunoreactivities were demonstrated in cytomegalic and heterotopic neurons of NMD rats. The results of the present study indicate that epileptogenesis of NMD might be caused by upregulation of glutamate receptor expression in dysplastic neurons of the rat cerebral cortex with NMDs.
Keyword
MeSH Terms
-
Animals
Cell Movement
Cerebral Cortex/*metabolism
Electroencephalography
Glutamic Acid/metabolism
Immunohistochemistry
Kainic Acid/pharmacology
Neurons/*metabolism/pathology
Rats
Rats, Wistar
Receptors, Glutamate/metabolism
Receptors, N-Methyl-D-Aspartate/*biosynthesis
Support, Non-U.S. Gov't
Time Factors
*Up-Regulation
Figure
Reference
-
1. Plate KH, Wieser HG, Yasargil MG, Wiestler OD. Neuropathological findings in 224 patients with temporal lobe epilepsy. Acta Neuropathol Berl. 1993. 86:433–438.
Article2. Wolf HK, Zentner J, Hufnagel A, Campos MG, Schramm J, Elger CE, Wiestler OD. Surgical pathology of chronic epileptic seizure disorders: Experience with 63 specimens from extratemporal corticectomies, lobectomies and functional hemispherectomies. Acta Neuropathol Berl. 1993. 86:466–472.
Article3. Lee MC, Kim GM, Woo YJ, Kim MK, Kim JH, Nam SC, Suh JJ, Chung WK, Lee JS, Kim HI, Choi HY, Kim SU. Pathogenic significance of neuronal migration disorders in temporal lobe epilepsy. Hum Pathol. 2001. 32:643–648.
Article4. Kuzniecky RI, Barkovich AJ. Pathogenesis and pathology of focal malformations of cortical development and epilepsy. J Clin Neurophysiol. 1996. 13:468–480.
Article5. Raymond AA, Fish DR, Sisodiya SM, Alsanjari N, Stevens JM, Shorvon SD. Abnormalities of gyration, heterotopias, tuberous sclerosis, focal cortical dysplasia, microdysgenesis, dysembryoplastic neuroepithelial tumour and dysgenesis of the archicortex in epilepsy. Clinical, EEG and neuroimaging features in 100 adult patients. Brain. 1995. 118:629–660.
Article6. Sohn EJ, Kim SJ, Lee MC, Kim HI. Neuropathologic studies of cerebral cortical dysplasia. J Korean Neurol Assoc. 1997. 15:526–541.7. Taylor DC, Falconer MA, Bruton CJ, Corsellis JAN. Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiat. 1971. 34:369–387.
Article8. Jacobs KM, Gutnick MJ, Price DA. Hyperexcitability in a model of cortical maldevelopment. Cereb Cortex. 1996. 6:514–523.
Article9. Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986. 233:1308–1311.
Article10. Roper SN. In utero irradiation of rats as a model of human cerebrocortical dysgenesis: a review. Epilepsy Res. 1998. 32:63–74.
Article11. Rosen GD, Press DM, Sherman GF, Galaburda AM. The development of induced cerebrocortical microgyria in the rat. J Neuropathol Exp Neurol. 1992. 51:601–611.
Article12. Baraban SC, Schwartzkroin PA. Electrophysiology of CA1 pyramidal neurons in an animal model of neuronal migration disorders: prenatal methylazoxymethanol treatment. Epilepsy Res. 1995. 22:145–156.
Article13. Sancini G, Franceschetti S, Battaglia G, Colacitti C, Di Luca M, Spreafico R, Avanzini G. Dysplastic neocortex and subcortical heterotopias in methylazoxymethanol-treated rats: an intracellular study of identified pyramidal neurones. Neurosci Lett. 1998. 246:181–185.
Article14. Ying Z, Babb TL, Comair YG, Bingaman W, Bushey M, Touhalisky K. Induced expression of NMDAR2 proteins and differential expression of NMDAR1 splice variants in dysplastic neurons of human epileptic neocortex. J Neuropathol Exp Neurol. 1998. 57:47–62.
Article15. Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981. 21:165–204.
Article16. Meldrum BS. Excitatory amino acids in epilepsy and potential novel therapies. Epilepsy Res. 1992. 12:189–196.
Article17. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroenceph Clin Neurophysiol. 1972. 32:281–294.
Article18. Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994. 17:31–108.
Article19. Nakanishi S, Masu M. Molecular diversity and functions of glutamate receptors. Annu Rev Biophys Biomol Struct. 1994. 23:319–348.
Article20. Lee MC, Rho JL, Kim MK, Woo YJ, Kim JH, Nam SC, Suh JJ, Chung WK, Moon JD, Kim HI. C-Jun expression and apoptotic cell death in Kainate-induced temporal lobe epilepsy. J Korean Med Sci. 2001. 16:649–656.
Article21. Hicks SP, D'Amato CJ, Lowe MJ. The development of the mammalian nervous system. I. Malformations of the brain, especially the cerebral cortex, induced in rats by radiation. II. Some mechanisms of the malformations of the cortex. J Comp Neurol. 1959. 113:435–469.
Article22. McGrath JJ, Riggs HE, Schwarz HP. Malformation of the adult brain (albino rat) resulting from prenatal irradiation. J Neuropathol Exp Neurol. 1956. 15:432–447.
Article23. Germano IM, Zhang YF, Sperber EF, Moshe SL. Neuronal migration disorders increase susceptibility to hyperthermia-induced seizures in developing rats. Epilepsia. 1996. 37:902–910.
Article24. Palmini A, Andermann F, Olivier A, Tampieri D, Robitaille Y, Andermann E, Wright G. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol. 1991. 30:741–749.
Article25. Germano IM, Sperber EF, Ahuja S, Moshe SL. Evidence of enhanced kindling and hippocampal neuronal injury in immature rats with neuronal migration disorders. Epilepsia. 1998. 39:1253–1260.26. Roper SN, King MA, Abraham LA, Boillot MA. Disinhibited in vitro neocortical slices containing experimentally induced cortical dysplasia demonstrate hyperexcitability. Epilepsy Res. 1997. 26:443–449.27. Mathern GW, Pretorius JK, Leite JP, Kornblum HI, Mendoza D, Lozada A, Bertram EH. 3rd Hippocampal AMPA and NMDA mRNA levels and subunit immunoreactivity in human temporal lobe epilepsy patients and a rodent model of chronic mesial limbic epilepsy. Epilepsy Res. 1998. 32:154–171.28. Mody I, Heinemann U. NMDA receptors of dentate gyrus granule cells participate in synaptic transmission following kindling. Nature. 1987. 326:701–704.
Article29. Babb TL, Ying Z, Hadam J, Penrod C. Glutamate receptor mechanisms in human epileptic dysplastic cortex. Epilepsy Res. 1998. 32:24–33.
Article30. Mikuni N, Babb TL, Ying Z, Najm I, Nishiyama K, Wylie C, Yacubova K, Okamoto T, Bingaman W. NMDA-receptors 1 and 2A/B coassembly increased in human epileptic focal cortical dysplasia. Epilepsia. 1999. 40:1683–1687.
Article31. Najm IM, Ying Z, Babb T, Mohamed A, Hadam J, LaPresto E, Wyllie E, Kotagal P, Bingaman W, Foldvary N, Morris H, Luders HO. Epileptogenicity correlated with increased N-methyl-D-aspartate receptor subunit NR2A/B in human focal cortical dysplasia. Epilepsia. 2000. 41:971–976.
Article32. Ying Z, Babb TL, Mikuni N, Najm I, Drazba J, Bingaman W. Selective coexpression of NMDAR2A/B and NMDAR1 subunit proteins in dysplastic neurons of human epileptic cortex. Exp Neurol. 1999. 159:409–418.
Article33. Lee MC. Normal cortical development and pathologic features of malformations of cortical development. J Korean Epilep Soc. 2000. 4:83–86.34. Mikuni N, Babb TL, Chakravarty DN, Hadam JL, Penrod CE. NMDAR2 upregulation precedes mossy fiber sprouting in kainate rat hippocampal epilepsy. Neurosci Lett. 1998. 255:25–28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Decrease of 14–3-3 proteins by glutamate exposure in the cerebral cortex of newborn rats
- Up-regulation of NMDA Receptor Subunit 2B Induces Degradation of Cytoskeletons in Hypoxic Rat Cerebral Cortex
- A study on the role of N-methyl-D-aspartate receptors in the rat visual cortex
- Decrease of protein phosphatase 2A subunit B by glutamate exposure in the cerebral cortex of neonatal rats
- The Study on the Role of NMDA Receptors in Rat Visual Cortex