Yonsei Med J.
2007 Dec;48(6):1009-1014. 10.3349/ymj.2007.48.6.1009.
Identification of Enhancer of Zeste Homolog 2 Expression in Peripheral Circulating Tumor Cells in Metastatic Prostate Cancer Patients: A Preliminary Study
- Affiliations
-
- 1Department of Urology, Urological Science Institute, Brain Korea 21 Project for Medical Science, College of Medicine, Korea. sjhong346@yuhs.ac
- 2Department of Food and Nutrition, Yonsei University, Seoul, Korea.
- KMID: 1786217
- DOI: http://doi.org/10.3349/ymj.2007.48.6.1009
Abstract
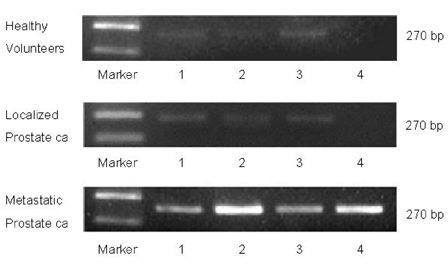
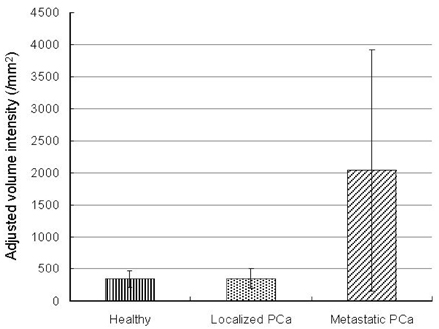
- PURPOSE: Enhancer of zeste homolog 2 (EZH2), a kind of transcriptional repressor, is reportedly over-expressed in metastatic prostate cancer. In this study, we analyzed EZH2 mRNA in circulating tumor cells (CTCs) in peripheral blood as a biomarker in patients with metastatic prostate cancer. PATIENTS AND METHODS: Ber-EP4 coated immunomagnetic beads were used to harvest CTCs, and mRNA was isolated by oligo- dT conjugated immunomagnetic beads. Reverse transcriptase- polymerase chain reaction for EZH2 mRNA was performed and the expression density was measured. The sensitivity of this test for detection of EZH2 mRNA was determined by serial dilutions of a human prostate cancer cell line. Blood samples were collected from 20 patients each with metastatic or localized prostate cancer and 10 healthy volunteers. RESULTS: Sensitivity experiments showed that the test was highly sensitive as it could detect 10 tumor cells per 5mL. EZH2 mRNA expression was obtained from peripheral blood samples of patients and control subjects. EZH2 mRNA expression density in the metastatic prostate cancer group was significantly higher than in the control (p=0.023) and localized prostate cancer groups (p=0.019). There was no difference between the control and localized prostate cancer groups (p > 0.05). CONCLUSION: EZH2 mRNA expression in circulating epithelial cells represents a promising marker for detecting early metastasis in prostate cancer. However, more specific and sensitive techniques for detection of CTCs are needed to avoid mononuclear cell contamination.
MeSH Terms
Figure
Reference
-
1. Corey E, Arfman EW, Oswin MM, Melchior SW, Tindall DJ, Young CY, et al. Detection of circulating prostate cells by reverse transcriptase-polymerase chain reaction of human glandular kallikrein (hK2) and prostate-specific antigen (PSA) messages. Urology. 1997. 50:184–188.2. Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003. 61:277–281.3. Ghossein RA, Scher HI, Gerald WL, Kelly WK, Curley T, Amsterdam A, et al. Detection of circulating tumor cells in patients with localized and metastatic prostatic carcinoma: clinical implications. J Clin Oncol. 1995. 13:1195–1200.4. Hara N, Kasahara T, Kawasaki T, Bilim V, Obara K, Takahashi K, et al. Reverse transcription-polymerase chain reaction detection of prostate-specific antigen, prostate-specific membrane antigen, and prostate stem cell antigen in one milliliter of peripheral blood: value for the staging of prostate cancer. Clin Cancer Res. 2002. 8:1794–1799.5. Israeli RS, Miller WH Jr, Su SL, Powell CT, Fair WR, Samadi DS, et al. Sensitive nested reverse transcription polymerase chain reaction detection of circulating prostatic tumor cells: comparison of prostate-specific membrane antigen and prostate-specific antigen-based assays. Cancer Res. 1994. 54:6306–6310.6. Katz AE, Olsson CA, Raffo AJ, Cama C, Perlman H, Seaman E, et al. Molecular staging of prostate cancer with the use of an enhanced reverse transcriptase-PCR assay. Urology. 1994. 43:765–775.7. Mejean A, Vona G, Nalpas B, Damotte D, Brousse N, Chretien Y, et al. Detection of circulating prostate derived cells in patients with prostate adenocarcinoma is an independent risk factor for tumor recurrence. J Urol. 2000. 163:2022–2029.8. Seiden MV, Kantoff PW, Krithivas K, Propert K, Bryant M, Haltom E, et al. Detection of circulating tumor cells in men with localized prostate cancer. J Clin Oncol. 1994. 12:2634–2639.9. Sokoloff MH, Tso CL, Kaboo R, Nelson S, Ko J, Dorey F, et al. Quantitative polymerase chain reaction does not improve preoperative prostate cancer staging: a clinicopathological molecular analysis of 121 patients. J Urol. 1996. 156:1560–1566.10. Wood DP Jr, Banerjee M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol. 1997. 15:3451–3457.11. Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002. 419:624–629.12. Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007. 67:547–556.13. Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, et al. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc Natl Acad Sci U S A. 2005. 102:12177–12182.14. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002. 167:528–534.15. Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001. 166:416–419.16. Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000. 164:101–105.17. Ghossein RA, Rosai J. Polymerase chain reaction in the detection of micrometastases and circulating tumor cells. Cancer. 1996. 78:10–16.18. Moreno JG, Croce CM, Fischer R, Monne M, Vihko P, Mulholland SG, et al. Detection of hematogenous micrometastasis in patients with prostate cancer. Cancer Res. 1992. 52:6110–6112.19. Moreno JG, O'Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, et al. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology. 2001. 58:386–392.20. Chen BT, Loberg RD, Neeley CK, O'Hara SM, Gross S, Doyle G, et al. Preliminary study of immunomagnetic quantification of circulating tumor cells in patients with advanced disease. Urology. 2005. 65:616–621.21. Ady N, Morat L, Fizazi K, Soria JC, Mathieu MC, Prapotnich D, et al. Detection of HER-2/neu-positive circulating epithelial cells in prostate cancer patients. Br J Cancer. 2004. 90:443–448.22. Saramäki OR, Tammela TL, Martikainen PM, Vessella RL, Visakorpi T. The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer. Genes Chromosomes Cancer. 2006. 45:639–645.23. Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex biomarker approach for determining risk of prostate-specific antigen-defined recurrence of prostate cancer. J Natl Cancer Inst. 2003. 95:661–668.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Docetaxel Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Mediated Apoptosis in Prostate Cancer Cells via Epigenetic Gene Regulation by Enhancer of Zeste Homolog 2
- Expression of Survivin Correlated with Antiapoptosis in Benign Prostate Hyperplasia and Prostate Cancer
- Circulating Tumor Cells: Detection Methods and Potential Clinical Application in Breast Cancer
- Circulating Tumor Cells and Extracellular Nucleic Acids in Breast Cancer
- First Korean Case of Weaver Syndrome Along with Neuroblastoma and Genetic Confirmation of EZH2 Variant