J Korean Med Sci.
2011 Jun;26(6):726-733. 10.3346/jkms.2011.26.6.726.
Mesenchymal Stem Cells Improve Wound Healing In Vivo via Early Activation of Matrix Metalloproteinase-9 and Vascular Endothelial Growth Factor
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Soonchunhyang University College of Medicine, Seoul, Korea.
- 2Department of Plastic and Reconstructive Surgery, Hanyang University Guri Hospital, Guri, Korea.
- 3Stem Cell Therapy Center and Institute for Clinical Molecular Biology Research, Soonchunhyang University College of Medicine, Seoul, Korea. jhwon@hosp.sch.ac.kr
- 4Department of Dermatology, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 1785956
- DOI: http://doi.org/10.3346/jkms.2011.26.6.726
Abstract
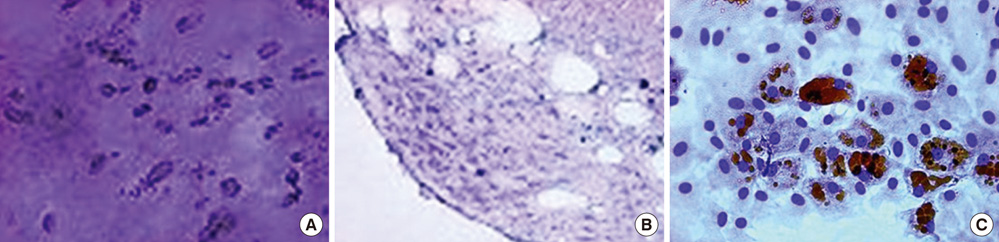
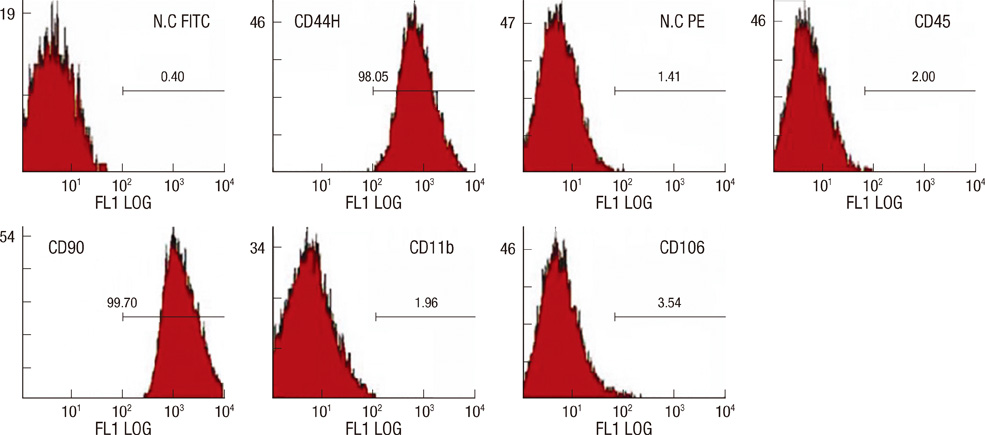
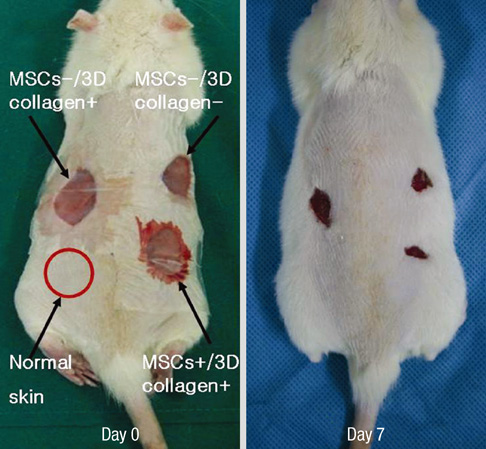
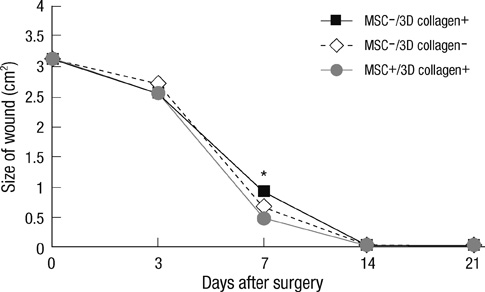
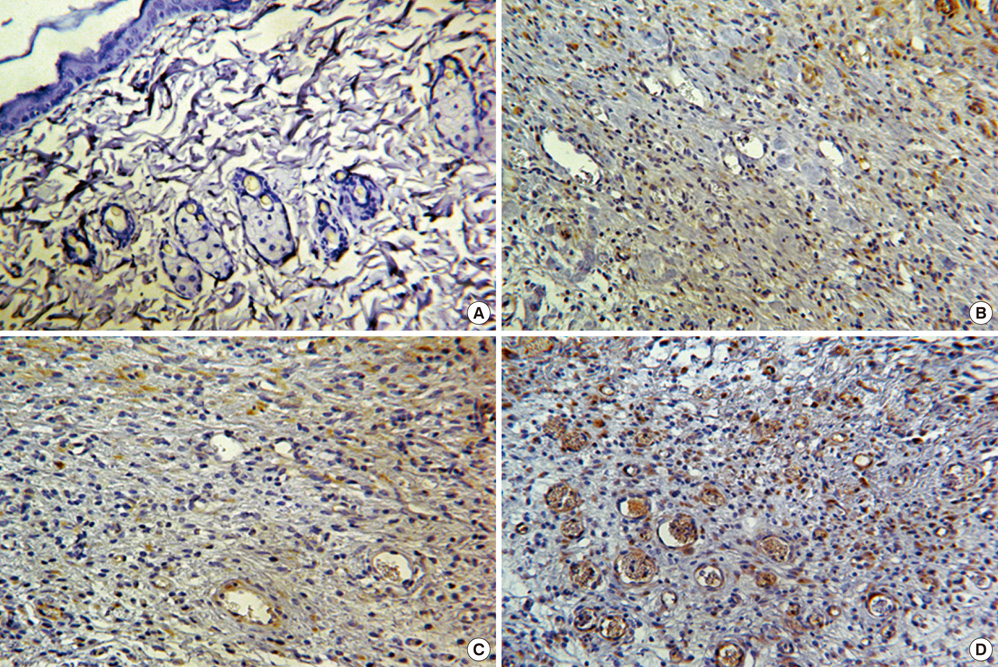
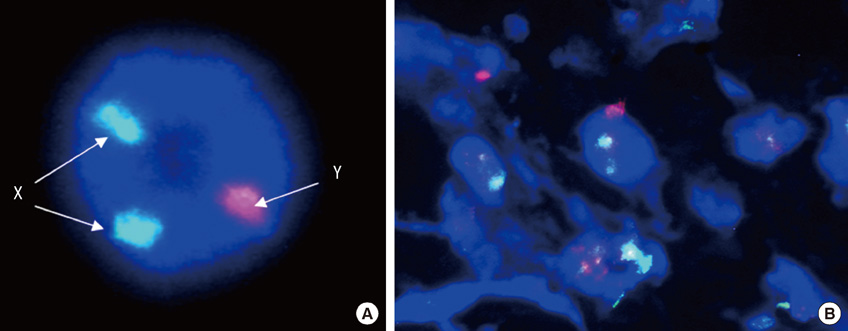
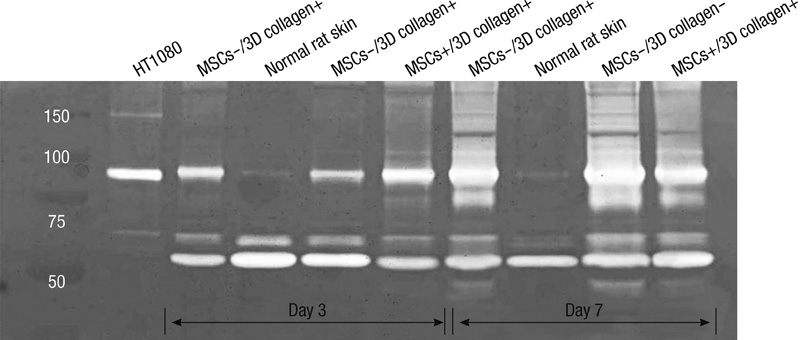
- We investigated the effects of mesenchymal stem cells (MSCs) on wound healing using a three-dimensional (3D) collagen gel scaffold. Three circular full-thickness skin defects were created on the back of Sprague-Dawley rats. One site was covered with a 3D collagen gel containing 2 x 10(6) MSCs (MSCs+/3D collagen+). Another site was replaced with a 3D collagen gel without MSCs and the third site was left empty. The wound size was significantly reduced in the MSCs+/3D collagen+ sites. MSCs+/3D collagen+ sites exhibited the most neovascularization. FISH showed that Y-chromosome possessing cells were found within the dermis of MSCs+/3D collagen+ sites. Gelatin zymography revealed that the most intense expression of MMP-9 was detected early in the MSCs+/3D collagen+ sites. Our results indicate that MSCs upregulate the early expression of MMP-9 which induces the early mobilization of VEGF. Thus, MSCs appear to accelerate significantly wound healing via early activation of MMP-9 and VEGF.
Keyword
MeSH Terms
Figure
Reference
-
1. Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997. 276:75–81.2. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999. 341:738–746.3. Vassalli JD, Saurat JH. Cuts and scrapes? Plasmin heals! Nat Med. 1996. 2:284–285.4. Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005. 65:3035–3039.5. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.6. Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005. 106:756–763.7. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.8. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999. 96:10711–10716.9. Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci USA. 2005. 102:4789–4794.10. Wu M, Yang L, Liu S, Li H, Hui N, Wang F, Liu H. Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue. Br J Dermatol. 2006. 155:282–291.11. Han SK, Yoon TH, Lee DG, Lee MA, Kim WK. Potential of human bone marrow stromal cells to accelerate wound healing in vitro. Ann Plast Surg. 2005. 55:414–419.12. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, Shrayer D, Carson P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007. 13:1299–1312.13. Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008. 40:1334–1347.14. Heissig B, Hattori K, Friedrich M, Rafii S, Werb Z. Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol. 2003. 10:136–141.15. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004. 109:1543–1549.16. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005. 11:367–368.17. Redmond DE Jr, Bjugstad KB, Teng YD, Ourednik V, Ourednik J, Wakeman DR, Parsons XH, Gonzalez R, Blanchard BC, Kim SU, Gu Z, Lipton SA, Markakis EA, Roth RH, Elsworth JD, Sladek JR Jr, Sidman RL, Snyder EY. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007. 104:12175–12180.18. Prockop DJ. "Stemness" does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther. 2007. 82:241–243.19. Flamme I, von Reutern M, Drexler HC, Syed-Ali S, Risau W. Overexpression of vascular endothelial growth factor in the avian embryo induces hypervascularization and increased vascular permeability without alterations of embryonic pattern formation. Dev Biol. 1995. 171:399–414.20. Hippenstiel S, Krüll M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998. 274:L678–L684.21. Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem. 1999. 274:31047–31054.22. Arnold F, West DC. Angiogenesis in wound healing. Pharmacol Ther. 1991. 52:407–422.23. Hollborn M, Stathopoulos C, Steffen A, Wiedemann P, Kohen L, Bringmann A. Positive feedback regulation between MMP-9 and VEGF in human RPE cells. Invest Ophthalmol Vis Sci. 2007. 48:4360–4367.24. Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007. 38:2563–2568.25. Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993. 4:1317–1326.26. Vincenti V, Cassano C, Rocchi M, Persico G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation. 1996. 93:1493–1495.27. Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991. 5:1806–1814.28. Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992. 267:26031–26037.29. Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005. 112:214–223.30. Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006. 14:840–850.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effective Delivering Method of Umbilical Cord Blood Stem Cells in Cutaneous Wound Healing
- Comparison of Bone Marrow Stromal Cells with Fibroblasts in Wound Healing Accelerating Growth Factor Secretion
- Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat
- Microarray Analysis of Gene Expression During Differentiation of Human Mesenchymal Stem Cells Treated with Vitamin E in vitro into Osteoblasts
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells