J Korean Med Sci.
2010 Sep;25(9):1352-1358. 10.3346/jkms.2010.25.9.1352.
Urodynamic and Histological Changes in a Sterile Rabbit Vesicoureteral Reflux Model
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Urology, Konkuk University Medical Center, Seoul, Korea.
- 3Department of Urology, Seoul National University Budang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Urology, Seoul National University College of Medicine and Clinical Research Institute, Seoul, Korea. hchoi@snu.ac.kr
- KMID: 1785916
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1352
Abstract
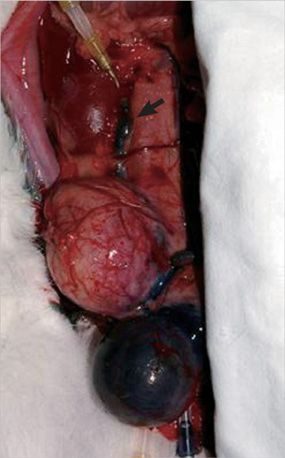
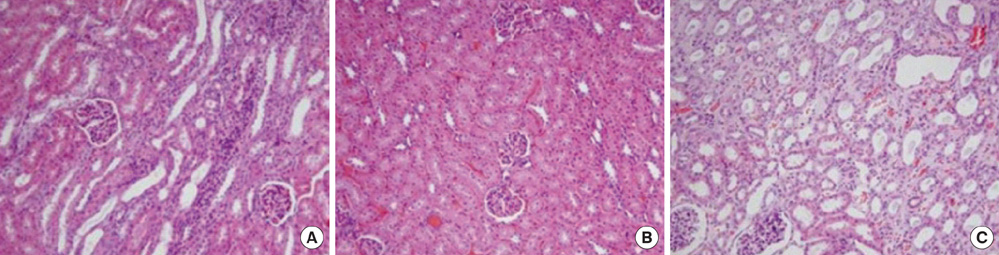
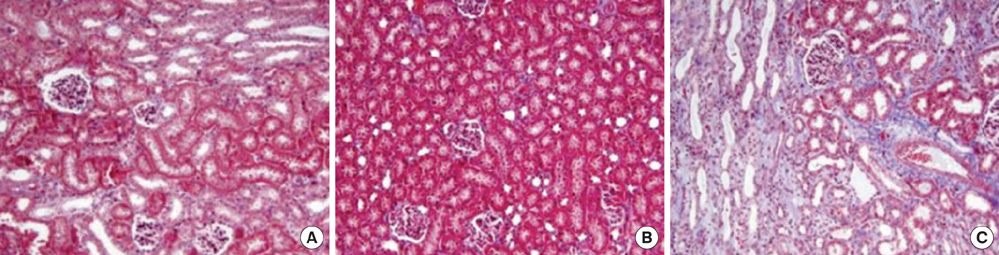
- This study aimed to investigate pressure changes of renal pelvis and histological change of kidneys in a surgically induced sterile rabbit vesicoureteral reflux (VUR) model. Five rabbits served as a control group, 7 as the sham-operated group, and 8 served as the VUR group. Three weeks later, urodynamic studies were performed, and histological examinations evaluated degree of inflammation, fibrosis, and tubular damage in the kidneys. At a low infusion rate, renal pelvic pressure in the VUR group was stable until late filling phase and then increased slightly. At a high infusion rate, the renal pelvic pressures of the sham-operated and control groups were stable until late filling phase and then increased slightly, whereas the renal pelvic pressure in the VUR group steadily increased from mid filling phase. Focal thinning of the tubular epithelium and interstitial widening were observed in certain cortical areas of refluxing kidneys, without inflammatory cell infiltration. Obvious changes in the mean diameters of distal tubules and extracellular matrix volume fractions were observed in two highly refluxing kidneys. High pressure reflux with bladder instability may result in renal cortical changes.
Keyword
MeSH Terms
Figure
Reference
-
1. King LR, Sellards HG. The effect of vesicoureteral reflux on renal growth and development in puppies. Invest Urol. 1971. 9:95–97.2. Lenaghan D, Cass AS, Cussen LJ, Stephens FD. Long-term effect of vesicoureteral reflux on the upper urinary tract of dogs. 1. Without urinary infection. J Urol. 1972. 107:755–757.3. Ransley PG, Risdon RA, Godley ML. Effects of vesicoureteric reflux on renal growth and function as measured by GFR, plasma creatinine and urinary concentrating ability. An experimental study in the minipig. Br J Urol. 1987. 60:193–204.
Article4. Angell SK, Pruthi RS, Shortliffe LD. The urodynamic relationship of renal pelvic and bladder pressures, and urinary flow rate in rats with congenital vesicoureteral reflux. J Urol. 1998. 160:150–156.
Article5. Okur H, Kose O, Kula M, Ozturk F, Muhtaroglu S, Sumerkan B. The role of infection and free oxygen radical damage in reflux nephropathy: an experimental study. J Urol. 2003. 169:1874–1877.
Article6. Smyth TB, Shortliffe LM, Constantinou CE. The effect of urinary flow and bladder fullness on renal pelvic pressure in a rat model. J Urol. 1991. 146:592–596.
Article7. Dewan PA, Ehall H, Edwards GA, MacGregor D. The development of a pig model to study fetal vesico-ureteric reflux. BJU Int. 2000. 86:1054–1057.
Article8. Gobet R, Cisek LJ, Chang B, Barnewolt CE, Retik AB, Peters CA. Experimental fetal vesicoureteral reflux induces renal tubular and glomerular damage, and is associated with persistent bladder instability. J Urol. 1999. 162:1090–1095.
Article9. Guvel S, Kilinc F, Kayaselcuk F, Egilmez T, Ozkardes H. Sterile vesicoureteral reflux decreases tubular cell apoptosis in rat kidney. Urology. 2005. 65:1244–1248.
Article10. Han CH, Kim SH, Kang SH, Shin OR, Lee HK, Kim HJ, Cho YH. Protective effects of cranberries on infection-induced oxidative renal damage in a rabbit model of vesico-ureteric reflux. BJU Int. 2007. 100:1172–1175.
Article11. Boyarsky A. The ureterovesical junction of the rabbit. Invest Urol. 1976. 13:383–384.12. Zinner NR, Paquin AJ Jr. Experimental vesicoureteral reflux. II. Roles of initial vesical volume, vesical capacity, vesical contractility, and cast-distance of the urinary stream. J Urol. 1963. 90:421–424.
Article13. Jorgensen TM, Stodkilde-Jorgensen H. Dynamics of the urinary tract during induced vesico-ureteric reflux in pigs. II. Scand J Urol Nephrol. 1985. 19:173–182.14. Mayo ME. Clinical experience with upper tract urodynamics. J Urol. 1983. 129:536–538.
Article15. Tanagho EA, Meyers FH, Smith DR. The trigone: anatomical and physiological considerations. I. In relation to the ureterovesical junction. J Urol. 1968. 100:623–632.16. Peterson AC, Webster GD. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Petersn CA, editors. Urodynamic and videourodynamic evaluation of voiding dysfunction. Campbell-Walsh Urology. 2007. 9th ed. Philadelphia: Saunders;1986–2010.17. Ransley PG, Risdon RA. Reflux nephropathy: effects of antimicrobial therapy on the evolution of the early pyelonephritic scar. Kidney Int. 1981. 20:733–742.
Article18. Smellie J, Edwards D, Hunter N, Normand IC, Prescod N. Vesico-ureteric reflux and renal scarring. Kidney Int Suppl. 1975. 4:S65–S72.