J Vet Sci.
2014 Jun;15(2):249-258. 10.4142/jvs.2014.15.2.249.
Equine hyperimmune serum protects mice against Clostridium difficile spore challenge
- Affiliations
-
- 1Animal Health Diagnostic Center, Departments of Population Medicine and Diagnostic Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA. yc42@cornell.edu
- 2Department of Bioscience Technology, Chang Jung Christian University, Tainan 71101, Taiwan R.O.C.
- 3Veterinary Institute, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, China.
- 4Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA.
- 5Department of Biomedical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853, USA.
- 6Lake Immunogenics Inc., Ontario, NY 14519, USA.
- KMID: 1784646
- DOI: http://doi.org/10.4142/jvs.2014.15.2.249
Abstract
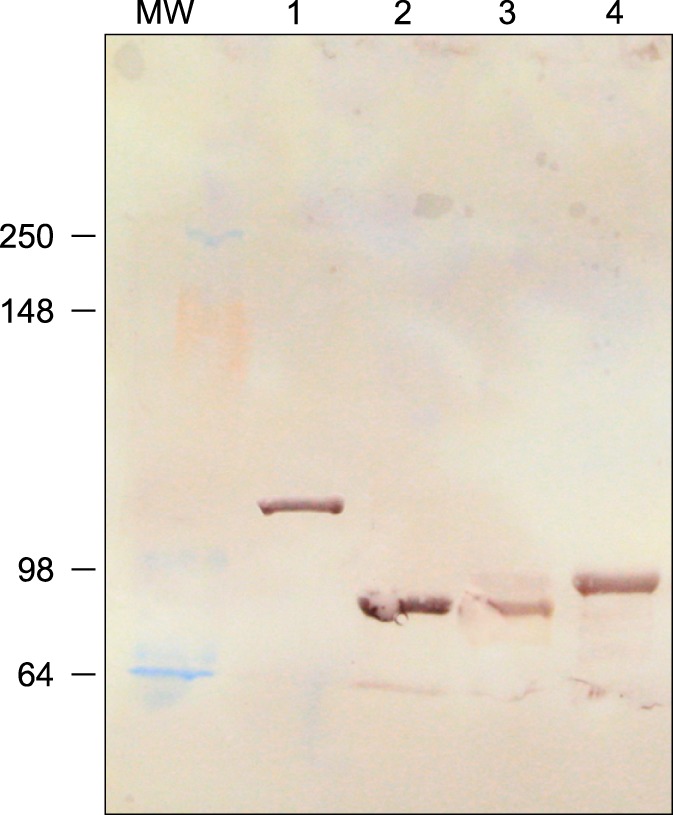
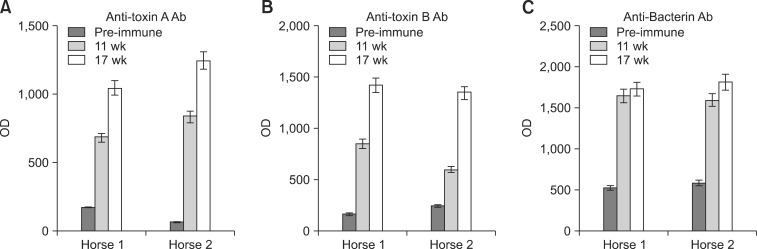
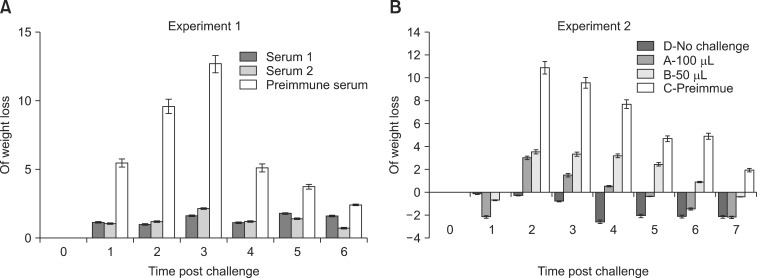
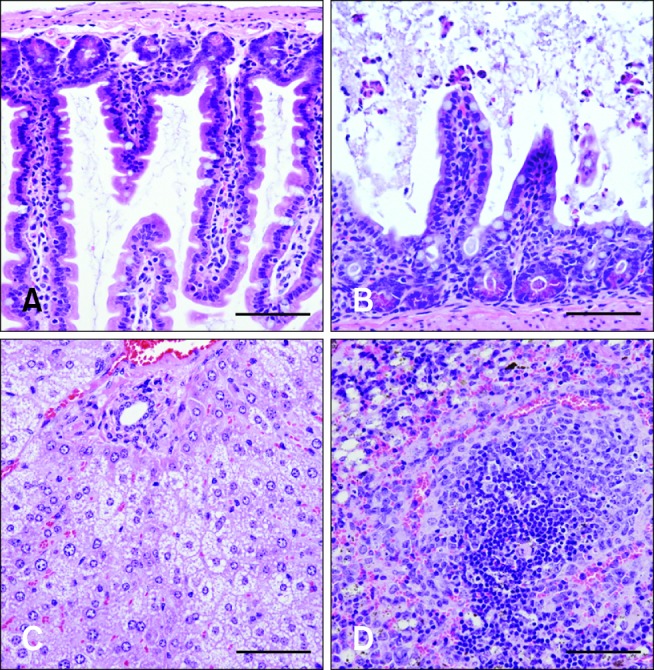
- Clostridium (C.) difficile is a common cause of nosocomial diarrhea in horses. Vancomycin and metronidazole have been used as standard treatments but are only moderately effective, which highlights the need for a novel alternative therapy. In the current study, we prepared antiserum of equine origin against both C. difficile toxins A and B as well as whole-cell bacteria. The toxin-neutralizing activities of the antibodies were evaluated in vitro and the prophylactic effects of in vivo passive immunotherapy were demonstrated using a conventional mouse model. The data demonstrated that immunized horses generated antibodies against both toxins A and B that possessed toxin-neutralizing activity. Additionally, mice treated with the antiserum lost less weight without any sign of illness and regained weight back to a normal range more rapidly compared to the control group when challenged orally with 10(7) C. difficile spores 1 day after serum injection. These results indicate that intravenous delivery of hyperimmune serum can protect animals from C. difficile challenge in a dose-dependent manner. Hence, immunotherapy may be a promising prophylactic strategy for preventing C. difficile infection in horses.
Keyword
MeSH Terms
-
Animals
Antibodies, Bacterial/blood/*immunology/therapeutic use
Bacterial Proteins/immunology/therapeutic use
Bacterial Toxins/immunology/therapeutic use
Clostridium Infections/microbiology/prevention & control/*veterinary
Clostridium difficile/*immunology
Enterotoxins/immunology/therapeutic use
Female
Horse Diseases/microbiology/*prevention & control
Horses
Immune Sera/*immunology
Immunization, Passive/*veterinary
Mice
Mice, Inbred C57BL
Spores, Bacterial/immunology
Antibodies, Bacterial
Bacterial Proteins
Bacterial Toxins
Enterotoxins
Immune Sera
Figure
Reference
-
1. Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011; 8:17–26. PMID: 21119612.2. Arroyo LG, Staempfli H, Weese JS. Molecular analysis of Clostridium difficile isolates recovered from horses with diarrhea. Vet Microbiol. 2007; 120:179–183. PMID: 17112686.3. Artiushin S, Timoney JF, Fettinger M, Fallon L, Rathgeber R. Immunisation of mares with binding domains of toxins A and B of Clostridium difficile elicits serum and colostral antibodies that block toxin binding. Equine Vet J. 2013; 45:476–480. PMID: 23206274.4. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002; 346:334–339. PMID: 11821511.5. Båverud V. Clostridium difficile diarrhea: infection control in horses. Vet Clin North Am Equine Pract. 2004; 20:615–630. PMID: 15519822.6. Båverud V, Franklin A, Gunnarsson A, Gustafsson A, Hellander-Edman A. Clostridium difficile associated with acute colitis in mares when their foals are treated with erythromycin and rifampicin for Rhodococcus equi pneumonia. Equine Vet J. 1998; 30:482–488. PMID: 9844966.7. Carman RJ, Stevens AL, Lyerly MW, Hiltonsmith MF, Stiles BG, Wilkins TD. Clostridium difficile binary toxin (CDT) and diarrhea. Anaerobe. 2011; 17:161–165. PMID: 21376825.8. Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. A mouse model of Clostridium difficile-associated disease. Gastroenterology. 2008; 135:1984–1992. PMID: 18848941.9. Corthier G, Muller MC, Wilkins TD, Lyerly D, L'Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991; 59:1192–1195. PMID: 1900059.10. Ehrich M, Van Tassell RL, Libby JM, Wilkins TD. Production of Clostridium difficile antitoxin. Infect Immun. 1980; 28:1041–1043. PMID: 7399686.11. Foglia G, Shah S, Luxemburger C, Pietrobon PJ. Clostridium difficile: development of a novel candidate vaccine. Vaccine. 2012; 30:4307–4309. PMID: 22682287.12. Forbes G, Church S, Savage CJ, Bailey SR. Effects of hyperimmune equine plasma on clinical and cellular responses in a low-dose endotoxaemia model in horses. Res Vet Sci. 2012; 92:40–44. PMID: 21093001.
Article13. Gardiner DF, Rosenberg T, Zaharatos J, Franco D, Ho DD. A DNA vaccine targeting the receptor-binding domain of Clostridium difficile toxin A. Vaccine. 2009; 27:3598–3604. PMID: 19464540.14. Giannasca PJ, Zhang ZX, Lei WD, Boden JA, Giel MA, Monath TP, Thomas WD Jr. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile disease in hamsters. Infect Immun. 1999; 67:527–538. PMID: 9916055.15. Giguère S, Gaskin JM, Miller C, Bowman JL. Evaluation of a commercially available hyperimmune plasma product for prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J Am Vet Med Assoc. 2002; 220:59–63. PMID: 12680449.16. Greenberg RN, Marbury TC, Foglia G, Warny M. Phase I dose finding studies of an adjuvanted Clostridium difficile toxoid vaccine. Vaccine. 2012; 30:2245–2249. PMID: 22306375.17. Johnson S, Gerding DN, Janoff EN. Systemic and mucosal antibody responses to toxin A in patients infected with Clostridium difficile. J Infect Dis. 1992; 166:1287–1294. PMID: 1431247.18. Keessen EC, Gaastra W, Lipman LJ. Clostridium difficile infection in humans and animals, differences and similarities. Vet Microbiol. 2011; 153:205–217. PMID: 21530110.19. Kelly CP, Pothoulakis C, Vavva F, Castagliuolo I, Bostwick EF, O'Keane JC, Keates S, LaMont JT. Anti-Clostridium difficile bovine immunoglobulin concentrate inhibits cytotoxicity and enterotoxicity of C. difficile toxins. Antimicrob Agents Chemother. 1996; 40:373–379. PMID: 8834883.20. Kink JA, Williams JA. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun. 1998; 66:2018–2025. PMID: 9573084.21. Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. The role of toxin A and toxin B in Clostridium difficile infection. Nature. 2010; 467:711–713. PMID: 20844489.22. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000; 342:390–397. PMID: 10666429.23. Leav BA, Blair B, Leney M, Knauber M, Reilly C, Lowy I, Gerding DN, Kelly CP, Katchar K, Baxter R, Ambrosino D, Molrine D. Serum anti-toxin B antibody correlates with protection from recurrent Clostridium difficile infection (CDI). Vaccine. 2010; 28:965–969. PMID: 19941990.24. Lyerly DM, Bostwick EF, Binion SB, Wilkins TD. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun. 1991; 59:2215–2218. PMID: 2037383.25. McFarland LV. Alternative treatments for Clostridium difficile disease: what really works? J Med Microbiol. 2005; 54:101–111. PMID: 15673502.26. Mulligan ME, Miller SD, McFarland LV, Fung HC, Kwok RY. Elevated levels of serum immunoglobulins in asymptomatic carriers of Clostridium difficile. Clin Infect Dis. 1993; 16(Suppl 4):S239–S244. PMID: 8324125.27. O'Brien JB, McCabe MS, Athié-Morales V, McDonald GS, Ní Eidhin DB, Kelleher DP. Passive immunisation of hamsters against Clostridium difficile infection using antibodies to surface layer proteins. FEMS Microbiol Lett. 2005; 246:199–205. PMID: 15899406.28. Oberli MA, Hecht ML, Bindschädler P, Adibekian A, Adam T, Seeberger PH. A possible oligosaccharide-conjugate vaccine candidate for Clostridium difficile is antigenic and immunogenic. Chem Biol. 2011; 18:580–588. PMID: 21609839.29. Ostrowski SR, Kubiski SV, Palmero J, Reilly CM, Higgins JK, Cook-Cronin S, Tawde SN, Crossley BM, Yant P, Cazarez R, Uzal FA. An outbreak of equine botulism type A associated with feeding grass clippings. J Vet Diagn Invest. 2012; 24:601–603. PMID: 22529134.
Article30. Peláez T, Alcalá L, Alonso R, Rodríguez-Créixems M, García-Lechuz JM, Bouza E. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob Agents Chemother. 2002; 46:1647–1650. PMID: 12019070.31. Roberts A, McGlashan J, Al-Abdulla I, Ling R, Denton H, Green S, Coxon R, Landon J, Shone C. Development and evaluation of an ovine antibody-based platform for treatment of Clostridium difficile infection. Infect Immun. 2012; 80:875–882. PMID: 22144483.32. Ruby R, Magdesian KG, Kass PH. Comparison of clinical, microbiologic, and clinicopathologic findings in horses positive and negative for Clostridium difficile infection. J Am Vet Med Assoc. 2009; 234:777–784. PMID: 19284345.33. Saito T, Kimura S, Tateda K, Mori N, Hosono N, Hayakawa K, Akasaka Y, Ishii T, Sumiyama Y, Kusachi S, Nagao J, Yamaguchi K. Evidence of intravenous immunoglobulin as a critical supportive therapy against Clostridium difficile toxin-mediated lethality in mice. J Antimicrob Chemother. 2011; 66:1096–1099. PMID: 21393125.34. Scaria J, Ponnala L, Janvilisri T, Yan W, Mueller LA, Chang YF. Analysis of ultra low genome conservation in Clostridium difficile. PLoS One. 2010; 5:e15147. PMID: 21170335.35. Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008; 190:2505–2512. PMID: 18245298.36. Tillotson GS, Tillotson J. Clostridium difficile-a moving target. F1000 Med Rep. 2011; 3:6. PMID: 21654927.37. Torres JF, Lyerly DM, Hill JE, Monath TP. Evaluation of formalin-inactivated Clostridium difficile vaccines administered by parenteral and mucosal routes of immunization in hamsters. Infect Immun. 1995; 63:4619–4627. PMID: 7591115.38. van Dissel JT, de Groot N, Hensgens CM, Numan S, Kuijper EJ, Veldkamp P, van't Wout J. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: preclinical and preliminary clinical data. J Med Microbiol. 2005; 54:197–205. PMID: 15673517.39. van Nispen Tot Pannerden CM, Verbon A, Kuipers EJ. Recurrent Clostridium difficile infection: what are the treatment options? Drugs. 2011; 71:853–868. PMID: 21568363.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SDS-PAGE profiles of clostridium difficile isolated from patientsand hospital environments
- New Treatment Option for Recurrent Clostridium difficile Infection
- Determination of toxin production of clostridium difficile strains isolated from patients with suspected antibiotic associated diarrhea
- Comparison of Clostridium difficile Toxin A Immunoassay with Cytotoxicity Assay
- A Case of Pseudomembranous Colitis due to Clostridium difficile