Neurointervention.
2013 Sep;8(2):92-100. 10.5469/neuroint.2013.8.2.92.
Computational Fluid Dynamics of Intracranial and Extracranal Arteries using 3-Dimensional Angiography: Technical Considerations with Physician's Point of View
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University Hospital, Seoul, Korea. stpark@schmc.ac.kr
- 2Department of Mechanical Engineering, Dankook University, Gyeonggido, Korea.
- 3Molds & Dies Technology R&D Group, Korea Institute of Industrial Technology, Incheon, Korea.
- 4Department of Radiology, University of Ulsan, College of Medicine, Asan Medical Center, Seoul, Korea.
- KMID: 1784025
- DOI: http://doi.org/10.5469/neuroint.2013.8.2.92
Abstract
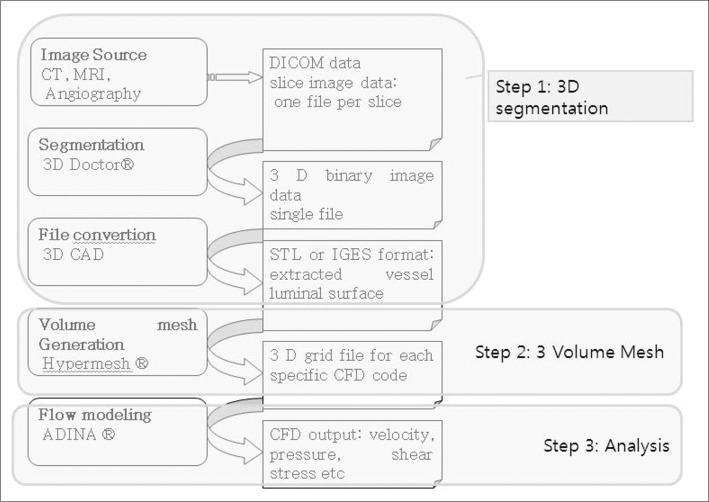
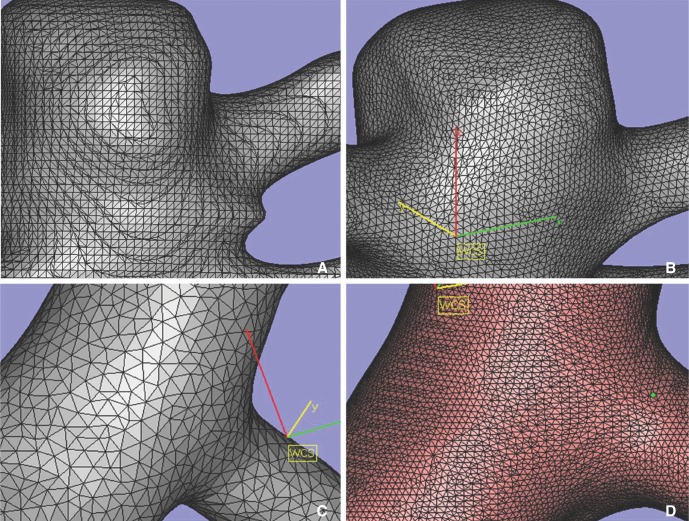
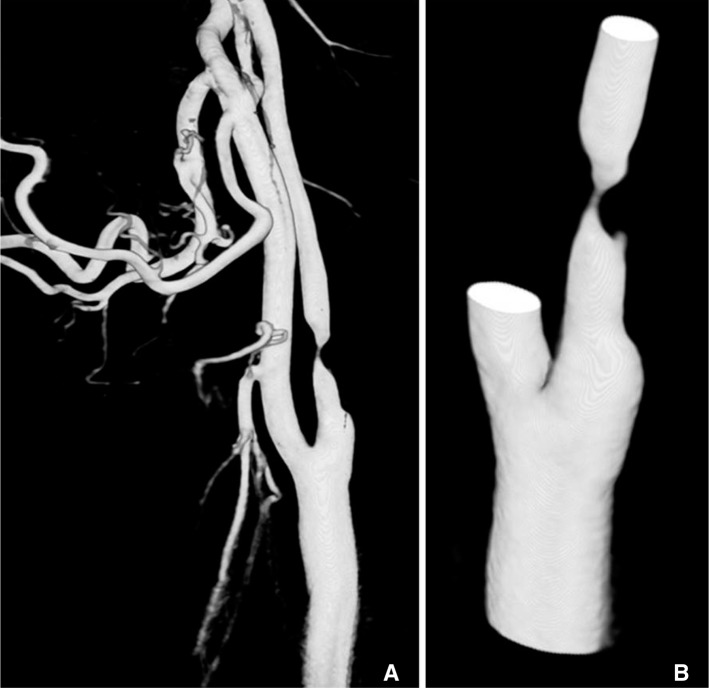
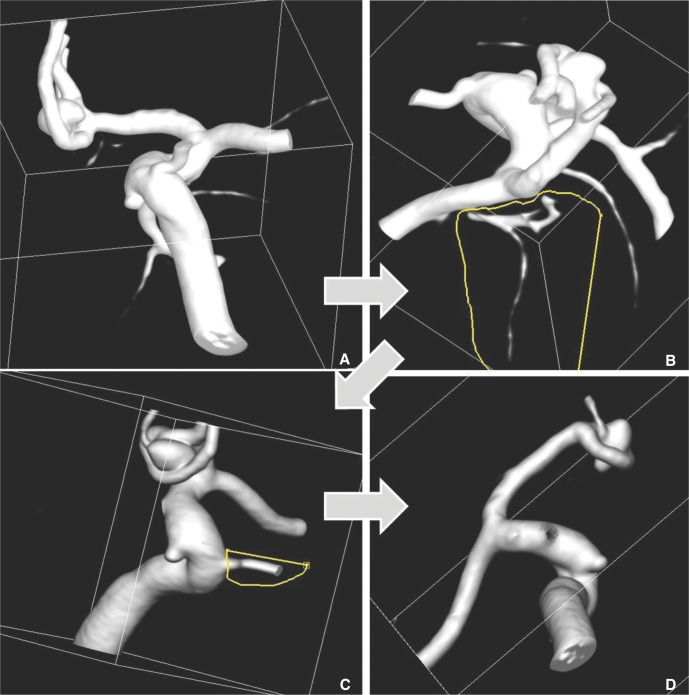
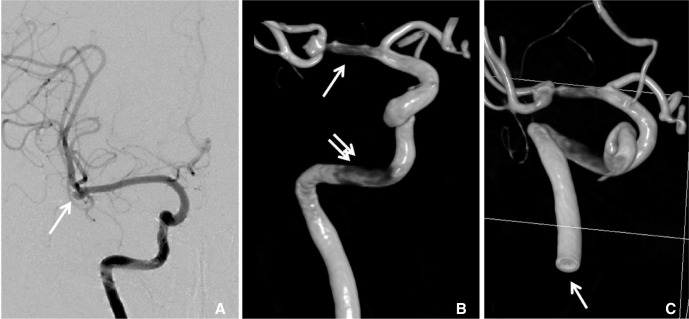
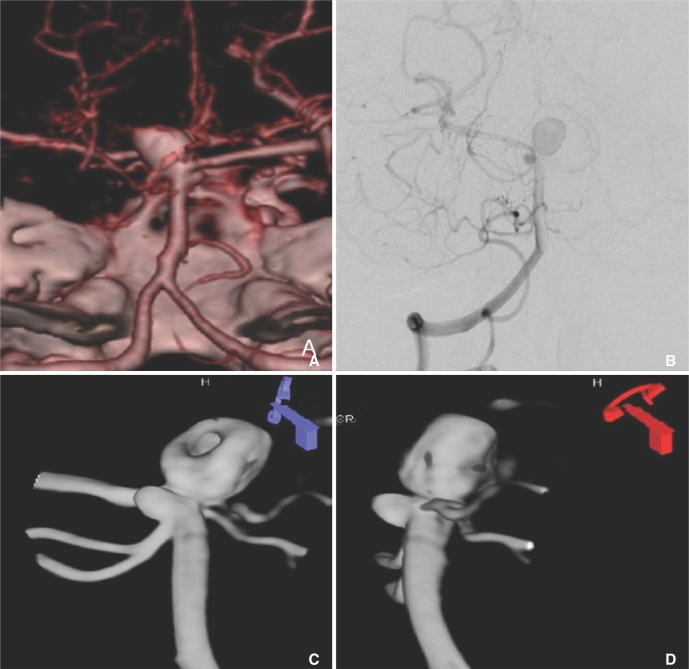
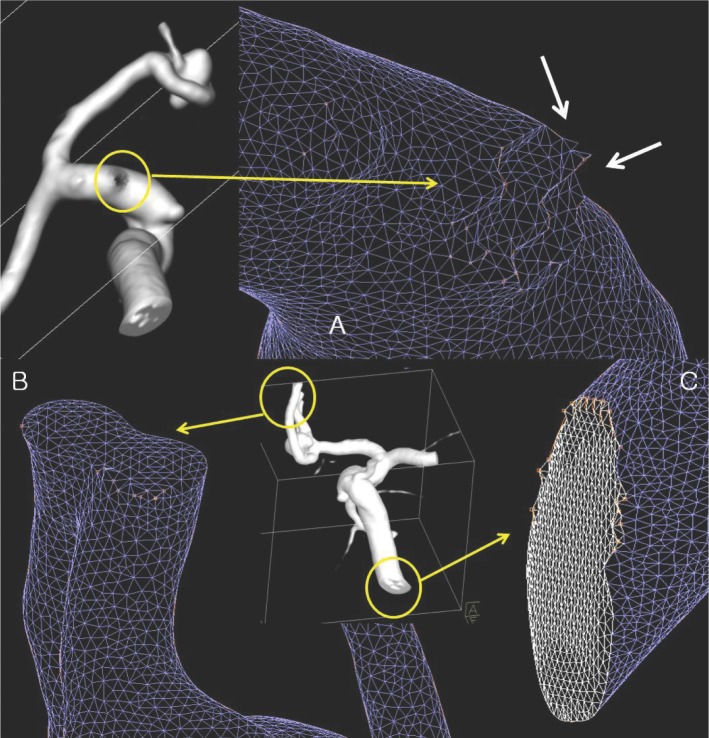
- We investigate the potentials and limitations of computational fluid dynamics (CFD) analysis of patient specific models from 3D angiographies. There are many technical problems in acquisition of proper vascular models, in pre-processing for making 2D surface and 3D volume meshes and also in post-processing steps for display the CFD analysis. We hope that our study could serves as a technical reference to validating other tools and CFD results.
Keyword
Figure
Reference
-
1. William R, Milnor MD. Hemodynamics. 2nd ed. Baltimore, Maryland: Williams & Wilkins;1989.2. Park S, Lee SW, Lim OK, Min I, Nguyen M, Ko YB, et al. Computational modeling with fluid-structure interaction of the severe m1 stenosis before and after stenting. Neurointervention. 2013; 8:23–28. PMID: 23515355.
Article3. Younis HF, Kaazempur-Mofrad MR, Chan RC, Isasi AG, Hinton DP, Chau AH, et al. Hemodynamics and wall mechanics in human carotid bifurcation and its consequences for atherogenesis: investigation of inter-individual variation. Biomech Model Mechanobiol. 2004; 3:17–32. PMID: 15300454.
Article4. Cebral JR, Mut F, Weir J, Putman CM. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol. 2011; 32:264–270. PMID: 21051508.
Article5. Suh DC, Park ST, Oh TS, Park SO, Lim OK, Park S, et al. High shear stress at the surface of enhancing plaque in the systolic phase is related to the symptom presentation of severe m1 stenosis. Korean J Radiol. 2011; 12:515–518. PMID: 21852914.
Article6. Nakatani H, Hashimoto N, Kang Y, Yamazoe N, Kikuchi H, Yamaguchi S, et al. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. J Neurosurg. 1991; 74:258–262. PMID: 1988596.
Article7. Park ST, Kim JK, Yoon KH, Park SO, Park SW, Kim JS, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. AJNR Am J Neuroradiol. 2010; 31:1106–1112. PMID: 20093309.
Article8. Goubergrits L, Thamsen B, Berthe A, Poethke J, Kertzscher U, Affeld K, et al. In vitro study of near-wall flow in a cerebral aneurysm model with and without coils. AJNR Am J Neuroradiol. 2010; 31:1521–1528. PMID: 20488901.
Article9. Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol. 2011; 32:27–33. PMID: 21071533.
Article10. Shaaban AM, Duerinckx AJ. Wall shear stress and early atherosclerosis: a review. AJR Am J Roentgenol. 2000; 174:1657–1665. PMID: 10845502.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Computational Fluid Dynamics of Intracranial Artery Using 3-Dimensional Angiography: Potentials and Technical Considerations
- Considerations of Blood Properties, Outlet Boundary Conditions and Energy Loss Approaches in Computational Fluid Dynamics Modeling
- Computational Fluid Dynamics in Three Dimensional Angiography: Preliminary Hemodynamic Results of Various Proximal Geometry
- Computational Fluid Dynamics: Comparison of Prototype and Commercial Solutions for Intracranial Aneurysms with Different Entrance Length
- Technical Consideration of Carotid Ophthalmic Aneurysms Surgery, Horizontal Medialward Directing Aneurysms Under the Optic Nerve: Report of Two Operative Cases