J Periodontal Implant Sci.
2010 Dec;40(6):257-264. 10.5051/jpis.2010.40.6.257.
Biological effects of a root conditioning agent for dentin surface modification in vitro
- Affiliations
-
- 1Research Institute, Nano Intelligent Biomedical Engineering Corporation (NIBEC), Seoul, Korea. ccpperio@snu.ac.kr
- 2Department of Periodontology and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- 3Department of Craniomaxillofacial Reconstuctive Science and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- KMID: 1783569
- DOI: http://doi.org/10.5051/jpis.2010.40.6.257
Abstract
- PURPOSE
Connective tissue reattachment to periodontally damaged root surfaces is one of the most important goals of periodontal therapy. The aim of this study was to develop a root conditioning agent that can demineralize and detoxify the infected root surface.
METHODS
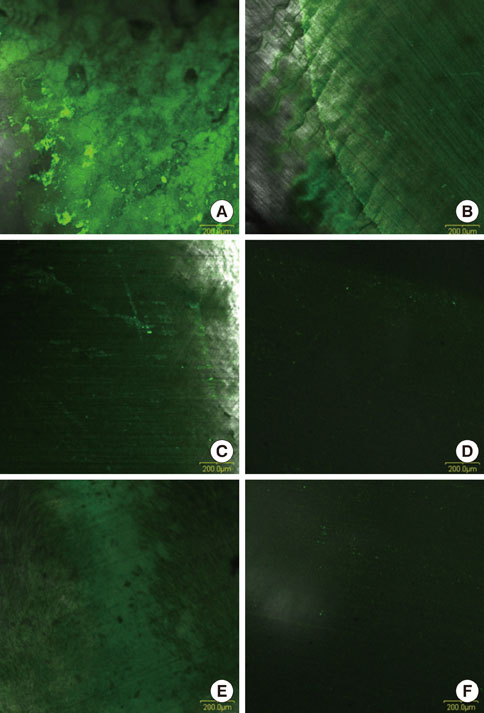
Dentin slices obtained from human teeth were treated with a novel root planing agent for 2 minutes and then washed with phosphate-buffered saline. Smear layer removal and type I collagen exposure were observed by scanning electron microscopy (SEM) and type I collagen immunostaining, respectively. Cell attachment and lipopolysaccharides (LPS) removal demonstrated the efficiency of the root conditioning agent.
RESULTS
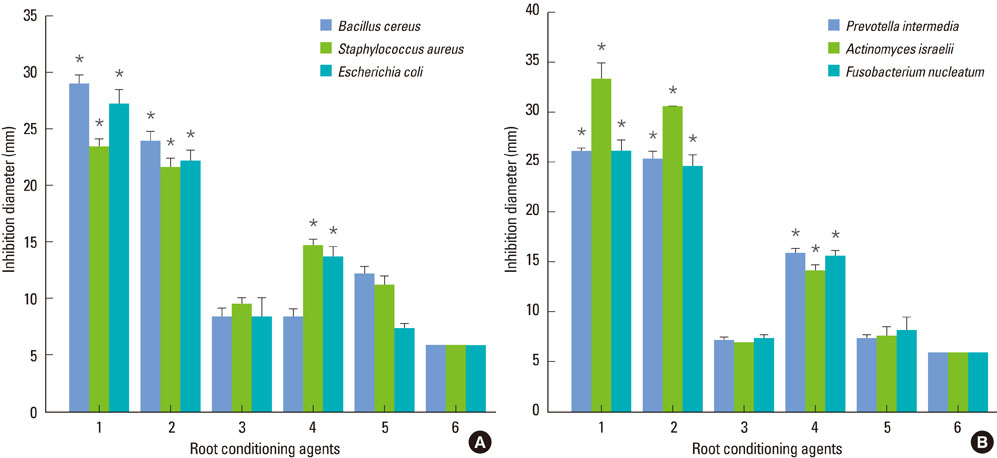
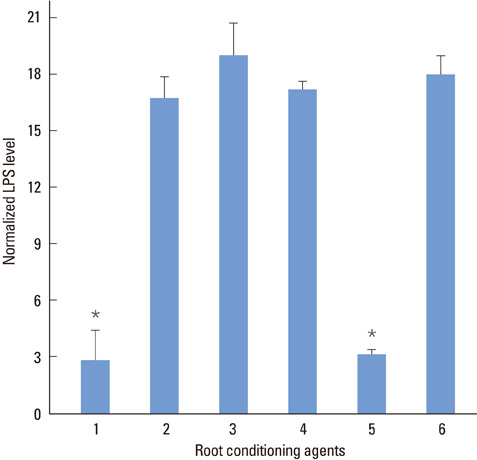
SEM revealed that the smear layer was entirely removed and the dentinal tubules were opened by the experimental gel. Type I collagen was exposed on the surfaces of the dentin slices treated by the experimental gel, which were compared with dentin treated with other root planing agents. Dentin slices treated with the experimental gel showed the highest number of attached fibroblasts and flattened cell morphology. The agar diffusion assay demonstrated that the experimental gel also has effective antimicrobial activity. Escherichia coli LPS were effectively removed from well plates by the experimental gel.
CONCLUSIONS
These results demonstrated that this experimental gel is a useful tool for root conditioning of infected root surfaces and can also be applied for detoxification of ailing implant surface threads.
MeSH Terms
Figure
Reference
-
1. Martorelli de Lima AF, Cury CC, Palioto DB, Duro AM, da Silva RC, Wolff LF. Therapy with adjunctive doxycycline local delivery in patients with type 1 diabetes mellitus and periodontitis. J Clin Periodontol. 2004. 31:648–653.
Article2. Nishimine D, O'Leary TJ. Hand instrumentation versus ultrasonics in the removal of endotoxins from root surfaces. J Periodontol. 1979. 50:345–349.
Article3. Baker PJ, Rotch HA, Trombelli L, Wikesjo UM. An in vitro screening model to evaluate root conditioning protocols for periodontal regenerative procedures. J Periodontol. 2000. 71:1139–1143.
Article4. Wikesjo UM, Selvig KA. Periodontal wound healing and regeneration. Periodontol 2000. 1999. 19:21–39.
Article5. Polson AM, Frederick GT, Ladenheim S, Hanes PJ. The production of a root surface smear layer by instrumentation and its removal by citric acid. J Periodontol. 1984. 55:443–446.
Article6. Isik G, Ince S, Saglam F, Onan U. Comparative SEM study on the effect of different demineralization methods with tetracycline HCl on healthy root surfaces. J Clin Periodontol. 1997. 24:589–594.
Article7. Babay N. The effect of EDTA on the attachment and growth of cultured human gingival fibroblasts in periodontitis-affected root surface. J Contemp Dent Pract. 2001. 2:13–23.8. Blomlof J, Lindskog S. Root surface texture and early cell and tissue colonization after different etching modalities. Eur J Oral Sci. 1995. 103:17–24.
Article9. Babay N. Attachment of human gingival fibroblasts to periodontally involved root surface following scaling and/or etching procedures: a scanning electron microscopy study. Braz Dent J. 2001. 12:17–21.10. Blomlof J, Lindskog S. Periodontal tissue-vitality after different etching modalities. J Clin Periodontol. 1995. 22:464–468.
Article11. Barkmeier WW, Jabro MH, Latta MA. Scanning electronic microscopic analysis of the local effects of a periodontal scaling gel on selected surfaces. J Clin Dent. 1992. 3:39–42.12. Chandra RV, Jagetia GC, Bhat KM. The attachment of V79 and human periodontal ligament fibroblasts on periodontally involved root surfaces following treatment with EDTA, citric acid, or tetracycline HCL: an SEM in vitro study. J Contemp Dent Pract. 2006. 7:44–59.
Article13. Rompen EH, Goffinet GH, Nusgens B. Human periodontal ligament fibroblast behavior on chemically conditioned dentine: an in vitro study. J Periodontol. 1999. 70:1144–1152.
Article14. Karp W, Sodek J, Aubin JE, Melcher AH. A comparison of fibronectin and laminin binding to undemineralized and demineralized tooth root surfaces. J Periodontal Res. 1986. 21:30–38.
Article15. McAllister B, Narayanan AS, Miki Y, Page RC. Isolation of a fibroblast attachment protein from cementum. J Periodontal Res. 1990. 25:99–105.
Article16. Egelberg J. Regeneration and repair of periodontal tissues. J Periodontal Res. 1987. 22:233–242.
Article17. Aktener BO, Bilkay U. Smear layer removal with different concentrations of EDTA-ethylenediamine mixtures. J Endod. 1993. 19:228–231.
Article18. Czonstkowsky M, Wilson EG, Holstein FA. The smear layer in endodontics. Dent Clin North Am. 1990. 34:13–25.19. Hulsmann M, Heckendorff M, Lennon A. Chelating agents in root canal treatment: mode of action and indications for their use. Int Endod J. 2003. 36:810–830.
Article20. Boveda C, Fajardo M, Millan B. Root canal treatment of an invaginated maxillary lateral incisor with a C-shaped canal. Quintessence Int. 1999. 30:707–711.21. Breschi L, Gobbi P, Lopes M, Prati C, Falconi M, Teti G, et al. Immunocytochemical analysis of dentin: a double-labeling technique. J Biomed Mater Res A. 2003. 67:11–17.
Article22. Pashley DH. Smear layer: physiological considerations. Oper Dent Suppl. 1984. 3:13–29.23. Blomlof J. Root cementum appearance in healthy monkeys and periodontitis-prone patients after different etching modalities. J Clin Periodontol. 1996. 23:12–18.
Article24. Larjava H, Salonen J, Hakkinen L, Narhi T. Effect of citric acid treatment on the migration of epithelium on root surfaces in vitro. J Periodontol. 1988. 59:95–99.
Article25. Perdigao J, Lambrechts P, Van Meerbeek B, Vanherle G, Lopes AL. Field emission SEM comparison of four postfixation drying techniques for human dentin. J Biomed Mater Res. 1995. 29:1111–1120.
Article26. Breschi L, Perdigao J, Gobbi P, Mazzotti G, Falconi M, Lopes M. Immunocytochemical identification of type I collagen in acid-etched dentin. J Biomed Mater Res A. 2003. 66:764–769.
Article27. Pitaru S, Aubin JE, Gray A, Metzger Z, Melcher AH. Cell migration attachment and orientation in vitro are enhanced by partial demineralization of dentine and cementum and inhibited by bacterial endotoxin. J Periodontal Res. 1984. 19:661–665.
Article28. Heritier M. Effects of phosphoric acid on root dentin surface. A scanning and transmission electron microscopic study. J Periodontal Res. 1984. 19:168–176.29. Wikesjo UM, Nilveus RE, Selvig KA. Significance of early healing events on periodontal repair: a review. J Periodontol. 1992. 63:158–165.
Article30. DePamphilis ML. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971. 105:1184–1199.
Article31. Yoshida T, Shibata T, Shinohara T, Gomyo S, Sekine I. Clinical evaluation of the efficacy of EDTA solution as an endodontic irrigant. J Endod. 1995. 21:592–593.
Article32. Daneshmand N, Jorgensen MG, Nowzari H, Morrison JL, Slots J. Initial effect of controlled release chlorhexidine on subgingival microorganisms. J Periodontal Res. 2002. 37:375–379.
Article33. Davies A. The mode of action of chlorhexidine. J Periodontal Res Suppl. 1973. 12:68–75.
Article34. Schnaitman CA. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971. 108:553–563.
Article35. Elin RJ, Wolff SM. Biology of endotoxin. Annu Rev Med. 1976. 27:127–141.
Article36. Dahlen G, Hofstad T. Endotoxic activities of lipopolysaccharides of microorganisms isolated from an infected root canal in Macaca cynomolgus. Scand J Dent Res. 1977. 85:272–278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Scanning Electron Microscopic Study of the Effect of EDTA on Demineralizing Diseased Root Surface
- Effect of tetracycline-HCl root conditioning on gingival epithelial cell attachment to root surface
- Effects of post surface conditioning before silanization on bond strength between fiber post and resin cement
- Effect of Tetracycline-HCL in Root Conditioning : A SEM Study
- The effects of dentin bonding agent thickness on stress distribution of composite-tooth interface : Finite element method