J Korean Med Sci.
2008 Oct;23(5):864-869. 10.3346/jkms.2008.23.5.864.
Efficient Cultivation Conditions for Human Limbal Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. wrwee@snu.ac.kr
- 2Seoul Artificial Eye Center, Seoul National University Hospital Clinical Research Institute, Seoul, Korea.
- 3Valued Eye Clinic, Daejeon, Korea.
- 4Laboratory of Tissue Engineering, Korea institute of Radiological and Medical Sciences, Seoul, Korea.
- KMID: 1783074
- DOI: http://doi.org/10.3346/jkms.2008.23.5.864
Abstract
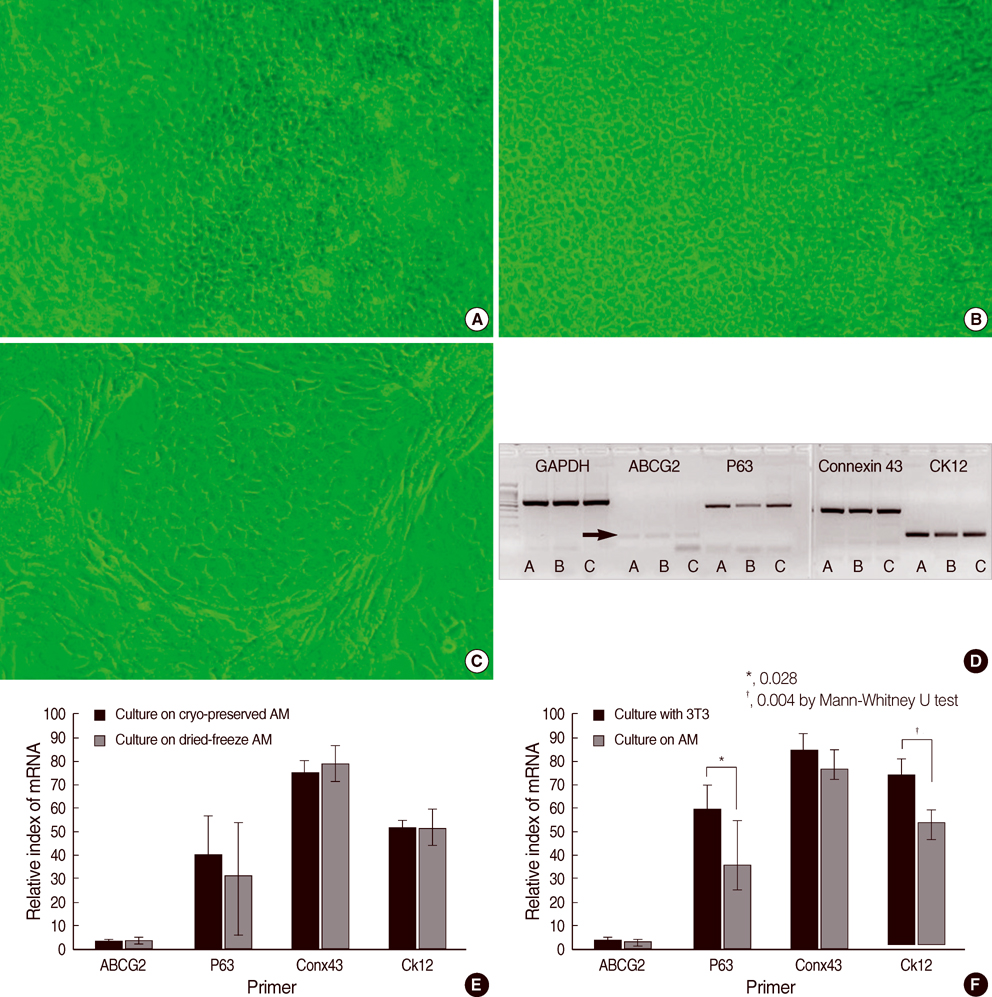
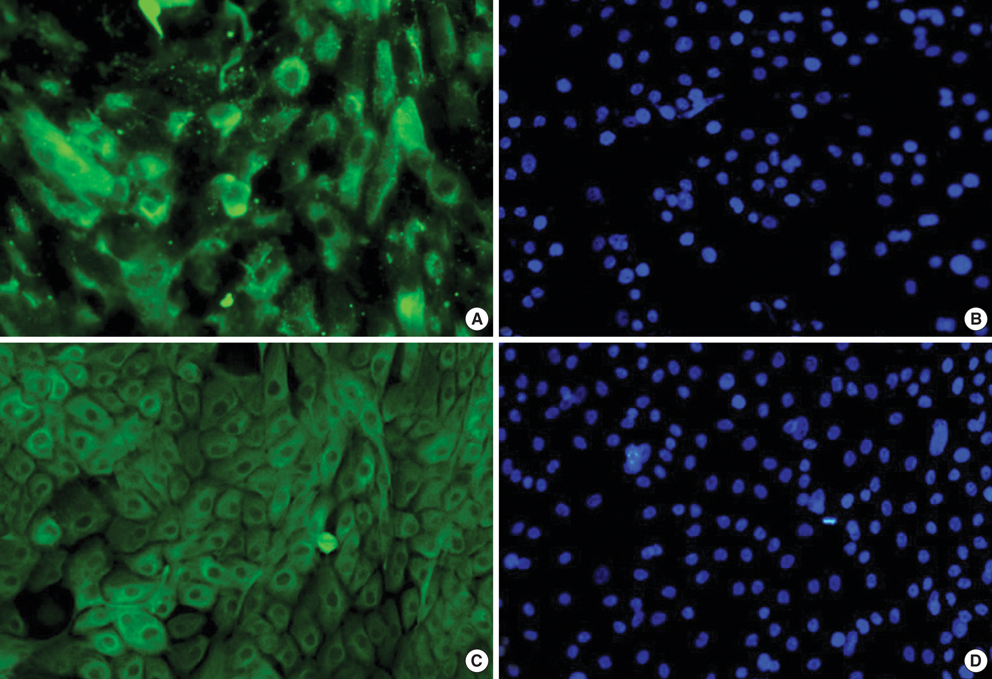
- To compare the stem niche in different culture conditions of limbal epithelial cells, the suspended human limbal epithelial cells (HLECs) were seeded on the 3T3-pretreated plates and the other suspended cells were plated on amniotic membranes (AMs) which were either cryo-preserved or freeze-dried. All were cultured for 10 to 12 days. Reverse transcription-polymerase chain reaction (RT-PCR) for ATP-binding casette, subfamily G, member 2 (ABCG2), p63, cytokeratin 12, and connexin 43 were performed in cultivated HLECs and their expression levels were compared. The mRNA expression of all markers examined showed no statistically significant differences between the cells on cryo-preserved and on freeze-dried AM. The expression of p63 and cytokeratin 12 in cultivated cells on AMs were significantly lower than those in 3T3-cocultured cells on RT-PCR and immunofluorescent staining. Cultivated HLECs on AMs showed reduced proliferation and differentiation while maintaining stem-property regardless of the preservative method of AM.
Keyword
MeSH Terms
-
3T3 Cells
Animals
Cell Culture Techniques/*instrumentation/*methods
Cells, Cultured
Cytological Techniques
DNA Primers/chemistry
Epithelial Cells/*metabolism
Humans
Immunohistochemistry/methods
Keratin-12/metabolism
Mice
Models, Biological
Phosphoproteins/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Stem Cells/cytology
Trans-Activators/metabolism
Figure
Reference
-
1. Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Nakamura T, Shimizu Y, Kinoshita S. Sterilized, freeze-dried AM: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004. 45:93–99.2. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986. 103:49–62.
Article3. Zieske JD. Perpetuation of stem cells in the eye. Eye. 1994. 8:163–169.
Article4. Chung EH, DeGregorio PG, Wasson M, Zieske JD. Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells. Invest Ophthalmol Vis Sci. 1995. 36:1336–1343.5. Pajoohesh-Ganji A, Ghosh SP, Stepp MA. Regional distribution of alpha9beta1 integrin within the limbus of the mouse ocular surface. Dev Dyn. 2004. 230:518–528.6. Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. P63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2004. 98:3156–3161.
Article7. Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005. 118:1715–1724.
Article8. Seigel GM, Sun W, Salvi R, Campbell LM, Sullivan S, Reidy JJ. Human corneal stem cells display functional neuronal properties. Mol Vis. 2003. 9:159–163.9. Zhao X, Das AV, Thoreson WB, James J, Wattnem TE, Rodriguez-Sierra J, Ahmad I. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002. 250:317–331.
Article10. Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004. 22:355–366.
Article11. Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004. 565:6–10.
Article12. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.
Article13. Zhang X, Sun H, Tang X, Ji J, Li X, Sun J, Ma Z, Yuan J, Han ZC. Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp Eye Res. 2005. 80:227–233.
Article14. Harkin DG, Barnard Z, Gillies P, Ainscough SL, Apel AJ. Analysis of p63 and cytokeratin expression in a cultivated limbal autograft used in the treatment of limbal stem cell deficiency. Br J Ophthalmol. 2004. 88:1154–1158.
Article15. Du Y, Chen J, Funderburgh JL, Zhu X, Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis. 2003. 9:635–643.16. Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003. 48:631–646.
Article17. Wang DY, Hsueh YJ, Yang VC, Chen JK. Propagation and phenotypic preservation of rabbit limbal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003. 44:4698–4704.
Article18. Grueterich M, Espana EM, Tseng SC. Modulation of keratin and connexin expression in limbal epithelium expanded on denuded amniotic membrane with and without a 3T3 fibroblast feeder layer. Invest Ophthalmol Vis Sci. 2003. 44:4230–4236.
Article19. Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003. 44:2959–2965.20. Ban Y, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Koizumi N, Dota A, Mochida C, Kinoshita S. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003. 76:735–743.
Article21. Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003. 76:303–314.
Article22. Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999. 18:73–79.
Article23. von Versen-Hoynck F, Syring C, Bachmann S, Moller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts--light and scanning electron microscopic studies. Cell Tissue Bank. 2004. 5:45–56.24. Limoli CL, Rola R, Giedzinski E, Mantha S, Huang TT, Fike JR. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci USA. 2004. 101:16052–16057.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of in vivo Cultivated Limbal Corneal Epithelial Cells with Total Limbal Stem Cell Deficiency
- The Characteristics of Limbal Epithelial Cells Auto-Cultivated In Vivo on Amniotic Membrane in Rabbits
- Long-term Outcome of Limbal Epithelial Cells Cultivated in Vivo on Amniotic Membrane Transplantation
- Comparison of Central Corneal Epithelial Healing Rates Between Different Wound Conditions
- Early Effect of Autologous Limbal Transplantation Immediately following Total Limbal Injury in Rabbits