Yonsei Med J.
2009 Feb;50(1):105-111. 10.3349/ymj.2009.50.1.105.
TGF-beta Mediated Epithelial-Mesenchymal Transition in Autosomal Dominant Polycystic Kidney Disease
- Affiliations
-
- 1Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. kyubeck.lee@samsung.com
- KMID: 1782975
- DOI: http://doi.org/10.3349/ymj.2009.50.1.105
Abstract
- PURPOSE
Recent studies have showed that epithelial-mesenchymal transition (EMT) is a key process of glomerular and tubulointerstitial pathology in many chronic kidney diseases. However, there are no data of EMT in humane autosomal dominant polycystic kidney disease (ADPKD).
PATIENTS AND METHODS
ADPKD kidneys (N = 5) with end stage renal disease (ESRD) and control kidneys (N = 4) were analyzed immnunohistochemically. We evaluated alpha-SMA, E-cadherin, vimentin, TGF-beta1 and Smad 2/3 expression in ADPKD and compared them with those in control kidney. These immunohistochemical findings were quantitatively analyzed by computer-assisted image analyzer and positive tubules (%).
RESULTS
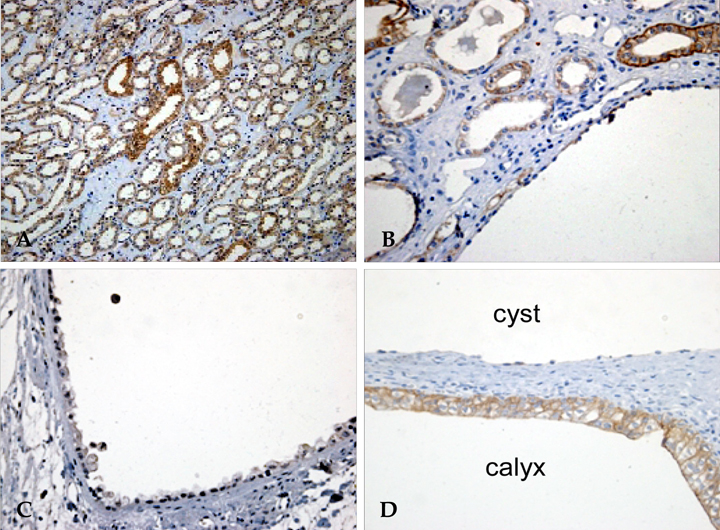
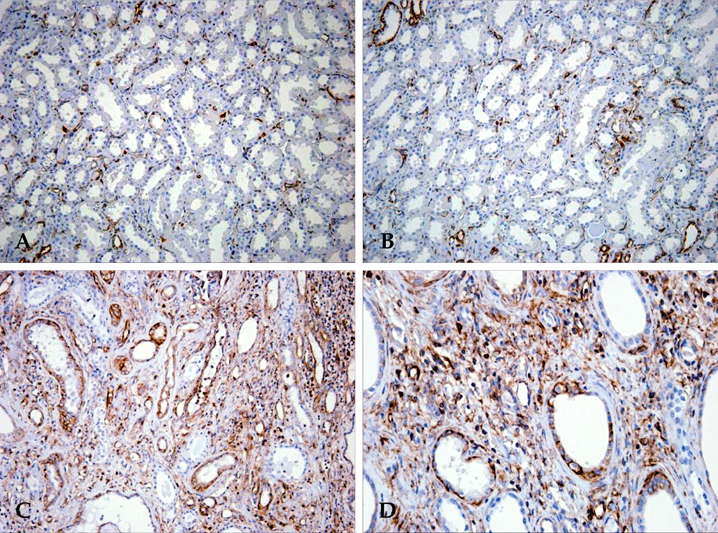
There were severe interstitial fibrosis and proliferation of alpha-SMA+ myofibroblasts in ADPKD. Cystic tubular epithelial cells in ADPKD lost epithelial marker (E-cadherin) and expressed mesenchymal markers (alpha-SMA, vimentin). There were significant increases of alpha-SMA (34.3 +/- 11.7% vs 0.9 +/- 1.5%), vimentin (19.9 +/- 3.9% vs 3.3 +/- 1.4%), TGF-beta1 (5.42 +/- 2.83% vs 0%) and Smad 2/3 (3.4 +/- 1.7% vs 0.7 +/- 0.6%) in ADPKD kidneys compared with control kidneys evidenced by computer-assisted image analyzer. When we analyze the positive tubules (%), the results were the same as computer-assisted image analyzer.
CONCLUSION
Our results showed that the end stage of ADPKD is associated with TGF-beta, Smad 2/3 and markers of EMT. It suggests that TGF-beta mediated EMT has a role in progression of ADPKD.
MeSH Terms
-
Aged
Biological Markers/metabolism
Cell Division
Disease Progression
Epithelial Cells/*pathology
Female
Fibrosis
Humans
Kidney Glomerulus/pathology
Kidney Tubules/pathology
Male
Mesoderm/*pathology
Middle Aged
Polycystic Kidney, Autosomal Dominant/*metabolism/*pathology
Transforming Growth Factor beta/*metabolism
Figure
Reference
-
1. Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005. 67:1234–1247.2. Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007. 71:846–854.3. Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006. 70:854–864.
Article4. Zeier M, Fehrenbach P, Geberth S, Möhring K, Waldherr R, Ritz E. Renal histology in polycystic kidney disease with incipient and advanced renal failure. Kidney Int. 1992. 42:1259–1265.
Article5. Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004. 15:1–12.
Article6. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002. 110:341–350.
Article7. Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001. 159:1465–1475.
Article8. Okada H, Ban S, Nagao S, Takahashi H, Suzuki H, Neilson EG. Progressive renal fibrosis in murine polycystic kidney disease: an immunohistochemical observation. Kidney Int. 2000. 58:587–597.
Article9. Schieren G, Rumberger B, Klein M, Kreutz C, Wilpert J, Geyer M, et al. Gene profiling of polycystic kidneys. Nephrol Dial Transplant. 2006. 21:1816–1824.
Article10. Böttinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002. 13:2600–2610.11. Wilson PD, Norman JT, Kuo NT, Burrow CR. Abnormalities in extracellular matrix regulation in autosomal dominant polycystic kidney disease. Contrib Nephrol. 1996. 118:126–134.12. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006. 7:131–142.13. Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005. 65:5991–5995. discussion 5995.
Article14. Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, et al. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int. 2002. 62:137–146.
Article15. Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, et al. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001. 38:761–769.
Article16. Yáñez-Mó M, Lara-Pezzi E, Selgas R, Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003. 348:403–413.
Article17. Vongwiwatana A, Tasanarong A, Rayner DC, Melk A, Halloran PF. Epithelial to mesenchymal transition during late deterioration of human kidney transplants: the role of tubular cells in fibrogenesis. Am J Transplant. 2005. 5:1367–1374.
Article18. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000. 342:1350–1358.
Article19. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006. 69:213–217.
Article20. Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005. 24:5764–5774.21. Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 2006. 17:19–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autosomal Dominant Polycystic Kidney Desease Coexisting with Renal Dysplasia. First Case Described and Followed Since Prenatal Period
- A Case of Renal Cell Carcinoma in Autosomal Dominant Polycystic Kidney Disease Hemodialyzed
- Apoptosis in Autosomal Dominant Polycystic Kidney Disease
- Autosomal Dominant Polycystic Kidney Desease Coexisting with Renal Dysplasia. First Case Described and Followed Since Prenatal Period
- Autosomal Dominant Polycystic Kidney Disease: 2009 Update for Internists