Yonsei Med J.
2009 Feb;50(1):60-67. 10.3349/ymj.2009.50.1.60.
Identification of Differentially Expressed Genes in Papillary Thyroid Cancers
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Pathology, Cancer Research Institute, Chungnam National University College of Medicine, Daejeon, Korea. jinmank@cnu.ac.kr
- KMID: 1782969
- DOI: http://doi.org/10.3349/ymj.2009.50.1.60
Abstract
- PURPOSE
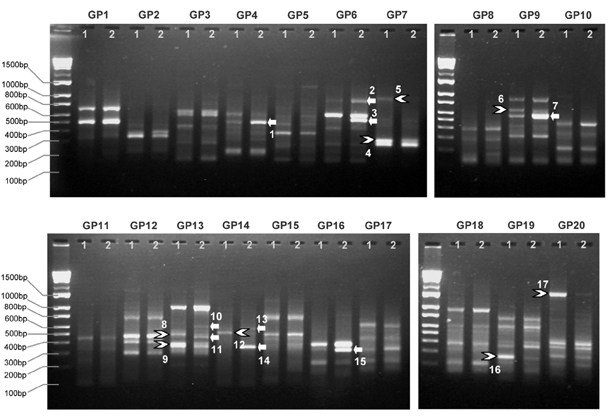
Techniques designed to identify differentially expressed genes (DEGs) in tumors have become important in modern pathology. Genefishing technique(TM) using the annealing control primer (ACP) system has recently been developed to screen for DEG transcripts. We tried to identify DEGs involved in papillary thyroid cancer (PTC) by using Genefishing technique(TM).
MATERIALS AND METHODS
We utilized a new differential display method, designated with Genefishing technique(TM), to analyze DEGs in 21 cases of PTCs.
RESULTS
Comparing the gene expression profiles between PTC and normal thyroid, we detected 17 genes that were differentially expressed in PTCs and performed cloning with sequencing in 10 genes. We confirmed the expression patterns of 2 DEGs by RT-PCR assay and identified the same results in 17 out of 21 (81%) PTCs. The 2 DEGs over-expressed in PTCs were identified as DC-STAMP and type I collagen A1. They are novel genes identified first in PTCs.
CONCLUSION
We confirmed 2 DEGs in PTCs as DC-STAMP and type I collagen A1 by using Genefishing technique(TM). Although the detailed functions of those 2 genes and their products remain to be determined, the genes will provide insights into mechanisms of carcinogenesis or tumor progression in PTCs.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Cytological Results of Ultrasound-Guided Fine-Needle Aspiration Cytology for Thyroid Nodules: Emphasis on Correlation with Sonographic Findings
Mi-Jung Lee, Soon Won Hong, Woung Youn Chung, Jin Young Kwak, Min Jung Kim, Eun-Kyung Kim
Yonsei Med J. 2011;52(5):838-844. doi: 10.3349/ymj.2011.52.5.838.
Reference
-
1. Choi YJ, Park YL, Koh JH. Prevalence of thyroid cancer at a medical screening center: pathological features of screen-detected thyroid carcinomas. Yonsei Med J. 2008. 49:748–756.
Article2. Inskip PD. Thyroid cancer after radiotherapy for childhood cancer. Med Pediatr Oncol. 2001. 36:568–573.
Article3. Inohara H, Honjo Y, Yoshii T, Akahani S, Yoshida J, Hattori K, et al. Expression of galectin-3 in fine-needle aspirates as a diagnostic marker differentiating benign from malignant thyroid neoplasms. Cancer. 1999. 85:2475–2484.
Article4. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. 417:949–954.
Article5. Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, Spiegelman BM, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science. 2000. 289:1357–1360.6. Ippolito A, Vella V, La Rosa GL, Pellegriti G, Vigneri R, Belfiore A. Immunostaining for Met/HGF receptor may be useful to identify malignancies in thyroid lesions classified suspicious at fine-needle aspiration biopsy. Thyroid. 2001. 11:783–787.
Article7. Casey MB, Lohse CM, Lloyd RV. Distinction between papillary thyroid hyperplasia and papillary thyroid carcinoma by immunohistochemical staining for cytokeratin 19, galectin-3, and HBME-1. Endocr Pathol. 2003. 14:55–60.
Article8. Hwang IT, Kim YJ, Kim SH, Kwak CI, Gu YY, Chun JY. Annealing control primer system for improving specificity of PCR amplification. Biotechniques. 2003. 35:1180–1184.
Article9. Yasui W, Oue N, Ito R, Kuraoka K, Nakayama H. Search for new biomarkers of gastric cancer through serial analysis of gene expression and its clinical implications. Cancer Sci. 2004. 95:385–392.
Article10. Nakoman C, Resmi H, Ay O, Acikel U, Atabey N, Güner G. ner G. Effects of basic fibroblast factor (bFGF) on MMP-2, TIMP-2, and type-I collagen levels in human lung carcinoma fibroblasts. Biochimie. 2005. 87:343–351.11. Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res. 2001. 61:1833–1838.12. St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000. 289:1197–1202.
Article13. Eleveld-Trancikova D, Triantis V, Moulin V, Looman MW, Wijers M, Fransen JA, et al. The dendritic cell-derived protein DC-STAMP is highly conserved and localizes to the endoplasmic reticulum. J Leukoc Biol. 2005. 77:337–343.
Article14. Hartgers FC, Vissers JL, Looman MW, van Zoelen C, Huffine C, Figdor CG, et al. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur J Immunol. 2000. 30:3585–3590.
Article15. Kukita T, Wada N, Kukita A, Kakimoto T, Sandra F, Toh K, et al. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J Exp Med. 2004. 200:941–946.
Article16. Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med. 2005. 202:345–351.
Article17. Hartgers FC, Looman MW, van der Woning B, Merkx GF, Figdor CG, Adema GJ. Genomic organization, chromosomal localization, and 5' upstream region of the human DC-STAMP gene. Immunogenetics. 2001. 53:145–149.
Article18. Fagin JA. Perspective: lessons learned from molecular genetic studies of thyroid cancer-insights into pathogenesis and tumor-specific therapeutic targets. Endocrinology. 2002. 143:2025–2028.
Article19. Bongarzone I, Pierotti MA. The molecular basis of thyroid epithelial tumorigenesis. Tumori. 2003. 89:514–516.
Article20. Nikiforova MN, Caudill CM, Biddinger P, Nikiforov YE. Prevalence of RET/PTC rearrangements in Hashimoto's thyroiditis and papillary thyroid carcinomas. Int J Surg Pathol. 2002. 10:15–22.
Article21. Santoro M, Papotti M, Chiappetta G, Garcia-Rostan G, Volante M, Johnson C, et al. RET activation and clinicopathologic features in poorly differentiated thyroid tumors. J Clin Endocrinol Metab. 2002. 87:370–379.
Article22. Tallini G. Molecular pathobiology of thyroid neoplasms. Endocr Pathol. 2002. 13:271–288.
Article23. Peyssonnaux C, Eychène A. The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell. 2001. 93:53–62.
Article24. Hilger RA, Scheulen ME, Strumberg D. The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie. 2002. 25:511–518.
Article25. Timler D, Tazbir J, Matejkowska M, Gosek A, Czyz W, Brzeziński J. Expression of proteins: D1 cyclin and Ki-67 in papillary thyroid carcinomas. Folia Histochem Cytobiol. 2001. 39 Suppl 2:201–202.26. Liang P, Averboukh L, Keyomarsi K, Sager R, Pardee AB. Differential display and cloning of messenger RNAs from human breast cancer versus mammary epithelial cells. Cancer Res. 1992. 52:6966–6968.27. Wan JS, Sharp SJ, Poirier GM, Wagaman PC, Chambers J, Pyati J, et al. Cloning differentially expressed mRNAs. Nat Biotechnol. 1996. 14:1685–1691.
Article28. Liang P, Pardee AB. Recent advances in differential display. Curr Opin Immunol. 1995. 7:274–280.
Article29. Zhao S, Ooi SL, Pardee AB. New primer strategy improves precision of differential display. Biotechniques. 1995. 18:842–846. 84885030. Nagy N, Decaestecker C, Dong X, Kaltner H, Schüring MP, Rocmans P, et al. Characterization of ligands for galectins, natural galactoside-binding immunoglobulin G subfractions and sarcolectin and also of the expression of calcyclin in thyroid lesions. Histol Histopathol. 2000. 15:503–513.31. Parmar DV, Khandkar MA, Pereira L, Bangur CS, Katyare SS. Thyroid hormones alter Arrhenius kinetics of succinate-2,6-dichloroindophenol reductase, and the lipid composition and membrane fluidity of rat liver mitochondria. Eur J Biochem. 1995. 230:576–581.32. Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci U S A. 2001. 98:15044–15049.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of differentially expressed genes using an annealing control primer system in periodontitis
- Idenfitied Differentially Expressed Genes in Keratoconus
- Expression of the MAGE-1, -2, -3, -4, -5, and -10 Genes in Thyroid Cancers
- Gene expression profiling of papillary thyroid carcinomas in Korean patients by oligonucleotide microarrays
- Pathologic basis of the sonographic differences between thyroid cancer and noninvasive follicular thyroid neoplasm with papillary-like nuclear features