Yonsei Med J.
2008 Dec;49(6):901-908. 10.3349/ymj.2008.49.6.901.
Short Insulin Tolerance Test Can Determine the Effects of Thiazolidinediones Treatment in Type 2 Diabetes
- Affiliations
-
- 1Department of Internal Medicine, 4Institute of Lifelong Health, Yonsei University Wonju College of Medicine, Wonju, Korea. cchung@yonsei.ac.kr
- 2Health Promotion Center, Samsung Seoul Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Endocrinology and Metabolism, Sun General Hospital, Daejeon, Korea.
- 4Institute of Lifelong Health, Yonsei University Wonju College of Medicine, Wonju, Korea.
- KMID: 1782937
- DOI: http://doi.org/10.3349/ymj.2008.49.6.901
Abstract
- PURPOSE
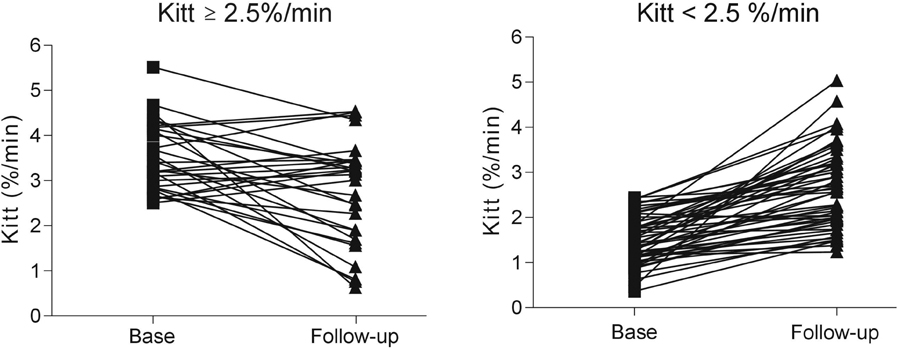
The short insulin tolerance test is a simple and reliable method of estimating insulin sensitivity. This study was designed to compare the insulin sensitizing effects of thiazolidinediones (TZDs) on the degree of insulin resistance, determined by a short insulin tolerance test (Kitt) in type 2 diabetic patients. PATIENTS AND METHODS: Eighty-three subjects (mean age = 57.87 +/- 10.78) with type 2 diabetes mellitus were enrolled and received daily one dose of rosiglitazone (4mg) or pioglitazone (15mg). The mean follow-up duration was 25.39 +/- 9.66 months. We assessed insulin sensitivity using HOMA-IR and the short insulin tolerance test before and after TZDs treatment. RESULTS: When we compared patients' characteristics before and after TZDs treatment, the mean fasting glucose level was significantly decreased (183.27 +/- 55.04 to 137.35 +/- 36.42mg/dL, p < 0.001) and the mean HbA1C level was significantly decreased (9.24 +/- 1.96 to 8.11 +/- 1.39%, p < 0.001). Also, Kitt values were significantly increased (2.03 +/- 1.14 to 2.67 +/- 0.97%/min, p = 0.003), whereas HOMA-IR was significantly decreased (2.98 +/- 0.68 to 1.04 +/- 0.24, p < 0.05). When classifying insulin resistance by Kitt values, insulin resistant subjects' values were increased (< 2.5%/min; 1.51 +/- 0.53%/min to 2.63 +/- 0.88, p < 0.001), whereas the values decreased in insulin sensitive subjects (> or = 2.5%/min; 3.50 +/- 0.75%/min to 2.75 +/- 1.12%/min, p = 0.002). CONCLUSION: The glucose lowering effects of TZDs by improving insulin resistance could be determined by using Kitt. However, Kitt may be a beneficial tool to determine TZDs' effects only when patients' Kitt values are less than 2.5%/min.
MeSH Terms
Figure
Cited by 1 articles
-
Association Between Serum Bilirubin and the Progression of Carotid Atherosclerosis in Type 2 Diabetes
Inkuk Lee, Hyeok-Hee Lee, Yongin Cho, Young Ju Choi, Byung Wook Huh, Byung-Wan Lee, Eun Seok Kang, Seok Won Park, Bong-Soo Cha, Eun Jig Lee, Yong-ho Lee, Kap Bum Huh
J Lipid Atheroscler. 2020;9(1):195-204. doi: 10.12997/jla.2020.9.1.195.
Reference
-
1. Lempiäinen P, Mykkänen L, Pyörälä K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999. 100:123–128.
Article2. Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996. 93:1809–1817.3. Haffner SM, D'Agostino R Jr, Mykkänen L, Tracy R, Howard B, Rewers M, et al. Insulin sensitivity in subjects with type 2 diabetes. Relationship to cardiovascular risk factors: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999. 22:562–568.
Article4. Morris AD, Ueda S, Petrie JR, Connell JM, Elliott HL, Donnelly R. The euglycaemic hyperinsulinaemic clamp: an evaluation of current methodology. Clin Exp Pharmacol Physiol. 1997. 24:513–518.
Article5. Inchiostro S. Measurement of insulin sensitivity in Type 2 diabetes mellitus: comparison between KITT and HOMA-%S indices and evaluation of their relationship with the components of the insulin resistance syndrome. Diabet Med. 2005. 22:39–44.6. Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, et al. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989. 68:374–378.
Article7. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995. 270:12953–12956.
Article8. Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996. 39:665–668.
Article9. Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, et al. A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci U S A. 1999. 96:6102–6106.
Article10. Park KS, Ciaraldi TP, Lindgren K, Abrams-Carter L, Mudaliar S, Nikoulina SE, et al. Troglitazone effects on gene expression in human skeletal muscle of type II diabetes involve up-regulation of peroxisome proliferator-activated receptor-gamma. J Clin Endocrinol Metab. 1998. 83:2830–2835.
Article11. Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu Rev Med. 2001. 52:239–257.
Article12. Park SW, Yun YS, Ahn CW, Nam JH, Kwon SH, Song MK, et al. Short insulin tolerance test (SITT) for the determination of in vivo insulin sensitivity-a comparison with euglycemic clamp test. J Korean Diabetes Assoc. 1998. 22:199–208.13. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.
Article14. Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003. 112:608–618.
Article15. Miyazaki Y, Matsuda M, De Fronzo RA. Dose-response effect of pioglitazone on insulin sensitivity and insulin secretion in type 2 diabetes. Diabetes Care. 2002. 25:517–523.
Article16. Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, et al. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001. 24:710–719.
Article17. Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002. 51:797–802.
Article18. Derosa G, D'Angelo A, Ragonesi PD, Ciccarelli L, Piccinni MN, Pricolo F, et al. Metformin-pioglitazone and metformin-rosiglitazone effects on non-conventional cardiovascular risk factors plasma level in type 2 diabetic patients with metabolic syndrome. J Clin Pharm Ther. 2006. 31:375–383.
Article19. Derosa G, Cicero AF, Gaddi A, Ragonesi PD, Fogari E, Bertone G, et al. Metabolic effects of pioglitazone and rosiglitazone in patients with diabetes and metabolic syndrome treated with glimepiride: a twelve-month, multicenter, double-blind, randomized, controlled, parallel-group trial. Clin Ther. 2004. 26:744–754.
Article20. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, De Fronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2007. 292:E871–E883.21. Matsui J, Terauchi Y, Kubota N, Takamoto I, Eto K, Yamashita T, et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-gamma-deficient mice on a high-fat diet. Diabetes. 2004. 53:2844–2854.
Article22. Shimabukuro M, Zhou YT, Lee Y, Unger RH. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J Biol Chem. 1998. 273:3547–3550.
Article23. Gastaldelli A, Miyazaki Y, Mahankali A, Berria R, Pettiti M, Buzzigoli E, et al. The effect of pioglitazone on the liver: role of adiponectin. Diabetes Care. 2006. 29:2275–2281.24. Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002. 51:2968–2974.
Article25. Miyazaki Y, He H, Mandarino LJ, DeFronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes. 2003. 52:1943–1950.
Article26. Osei K, Gaillard T, Schuster D. Thiazolidinediones increase hepatic insulin extraction in African Americans with impaired glucose tolerance and type 2 diabetes mellitus. A pilot study of rosiglitazone. Metabolism. 2007. 56:24–29.
Article27. Jones TA, Sautter M, Van Gaal LF, Jones NP. Addition of rosiglitazone to metformin is most effective in obese, insulin-resistant patients with type 2 diabetes. Diabetes Obes Metab. 2003. 5:163–170.
Article28. Radikova Z. Assessment of insulin sensitivity/resistance in epidemiological studies. Endocr Regul. 2003. 37:189–194.29. Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Funahashi T, et al. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007. 77:151–154.
Article30. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004. 27:1487–1495.
Article31. Akinmokun A, Selby PL, Ramaiya K, Alberti KG. The short insulin tolerance test for determination of insulin sensitivity: a comparison with the euglycaemic clamp. Diabet Med. 1992. 9:432–437.
Article32. Hirst S, Phillips DI, Vines SK, Clark PM, Hales CN. Reproducibility of the short insulin tolerance test. Diabet Med. 1993. 10:839–842.
Article33. Grulet H, Durlach V, Hecart AC, Gross A, Leutenegger M. Study of the rate of early glucose disappearance following insulin injection: insulin sensitivity index. Diabetes Res Clin Pract. 1993. 20:201–207.
Article34. Robertson RP, Zhang HJ, Pyzdrowski KL, Walseth TF. Preservation of insulin mRNA levels and insulin secretion in HIT cells by avoidance of chronic exposure to high glucose concentrations. J Clin Invest. 1992. 90:320–325.
Article35. Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am J Physiol. 1996. 270:E251–E258.
Article36. Andrews WJ, Vasquez B, Nagulesparan M, Klimes I, Foley J, Unger R, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984. 33:634–642.
Article37. Garver WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985. 34:222–234.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Resistance and Insulin Resistance Syndrome
- The Effect of Growth Hormone on Carbohydrate Metabolism in Turner Syndrome
- Effects of growth hormone treatment on glucose metabolism in idiopathic short stature
- Diagnosis and Glycemic Control of Type 1 Diabetes
- Triple Combination Therapy Using Metformin, Thiazolidinedione, and a GLP-1 Analog or DPP-IV Inhibitor in Patients with Type 2 Diabetes Mellitus