J Korean Med Sci.
2011 Feb;26(2):290-296. 10.3346/jkms.2011.26.2.290.
Morphine Postconditioning Attenuates ICAM-1 Expression on Endothelial Cells
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. minware2@lycos.co.kr
- 2Department of Biomedical Sciences, Graduate School of Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 1782121
- DOI: http://doi.org/10.3346/jkms.2011.26.2.290
Abstract
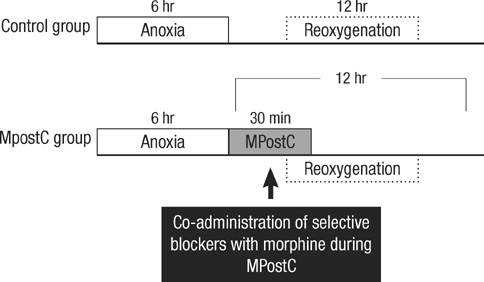
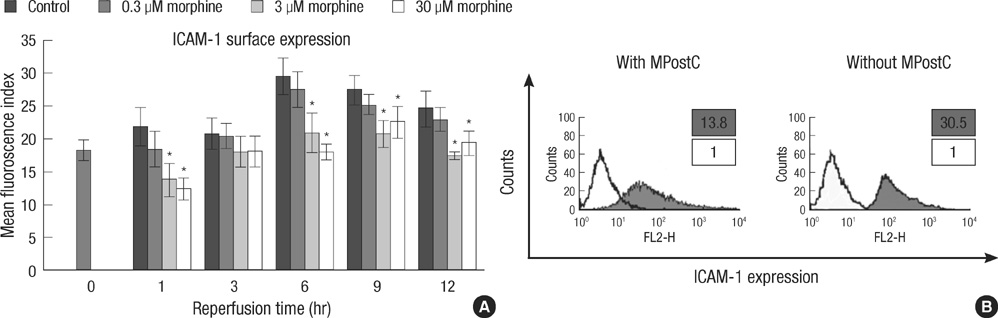
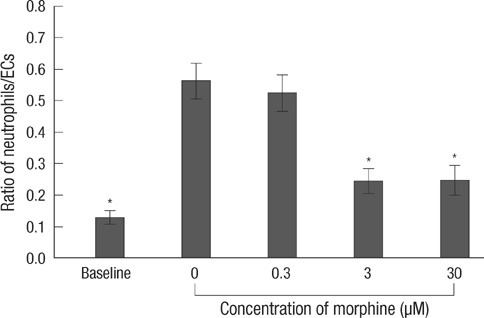
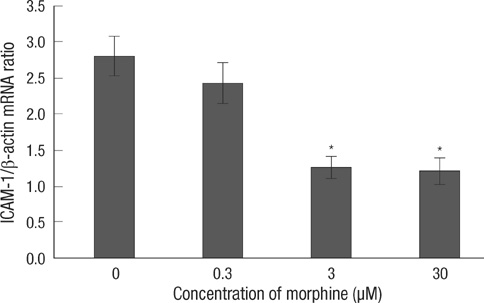
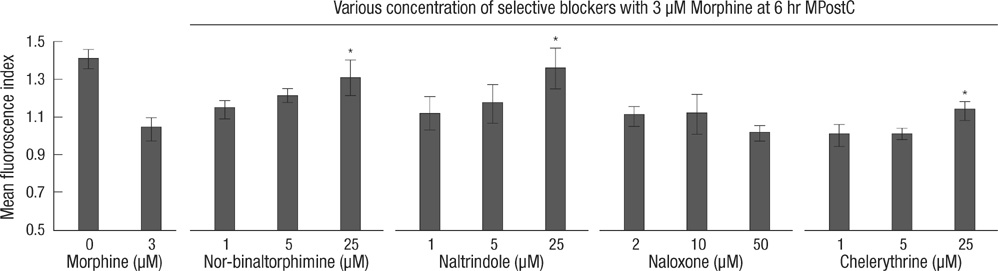
- The purpose of this study is to determine 1) whether morphine postconditiong (MPostC) can attenuate the intercellular adhesion molecules-1 (ICAM-1) expression after reoxygenation injury and 2) the subtype(s) of the opioid receptors (ORs) that are involved with MPostC. Human umbilical vein endothelial cells (HUVECs) were subjected to 6 hr anoxia followed by 12 hr reoxygenation. Three morphine concentrations (0.3, 3, 30 microM) were used to evaluate the protective effect of MPostC. We also investigated blockading the OR subtypes' effects on MPostC by using three antagonists (a micro-OR antagonist naloxone, a kappa-OR antagonist nor-binaltorphimine, and a delta-OR antagonist naltrindole) and the inhibitor of protein kinase C (PKC) chelerythrine. As results, the ICAM-1 expression was significantly reduced in the MPostC (3, 30 microM) groups compared to the control group at 1, 6, 9, and 12 hours reoxygenation time. As a consequence, neutrophil adhesion was also decreased after MPostC. These effects were abolished by coadministering chelerythrine, nor-binaltorphimine or naltrindole, but not with naloxone. In conclusion, it is assumed that MPostC could attenuate the expression of ICAM-1 on endothelial cells during reoxygenation via the kappa and delta-OR (opioid receptor)-specific pathway, and this also involves a PKC-dependent pathway.
Keyword
MeSH Terms
-
Animals
Benzophenanthridines/pharmacology
Endothelial Cells/cytology/*drug effects/*metabolism
Endothelium, Vascular/cytology
Humans
Intercellular Adhesion Molecule-1/genetics/*metabolism
Morphine/*pharmacology
Naloxone/pharmacology
Naltrexone/analogs & derivatives/pharmacology
Narcotic Antagonists/pharmacology
Narcotics/*pharmacology
Protein Isoforms/metabolism
Protein Kinase C/antagonists & inhibitors/metabolism
Receptors, Opioid/metabolism
Reperfusion Injury/*metabolism
Signal Transduction/physiology
Umbilical Veins/cytology
Figure
Reference
-
1. Ma XL, Tsao PS, Lefer AM. Antibody to CD-18 exerts endothelial and cardiac protective effects in myocardial ischemia and reperfusion. J Clin Invest. 1991. 88:1237–1243.2. Weyrich AS, Ma XY, Lefer DJ, Albertine KH, Lefer AM. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993. 91:2620–2629.3. Kukielka GL, Hawkins HK, Michael L, Manning AM, Youker K, Lane C, Entman ML, Smith CW, Anderson DC. Regulation of intercellular adhesion molecule-1 (ICAM-1) in ischemic and reperfused canine myocardium. J Clin Invest. 1993. 92:1504–1516.4. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003. 285:H579–H588.5. Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005. 67:124–133.6. Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004. 78:961–969.7. Sun HY, Wang NP, Halkos M, Kerendi F, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Postconditioning attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Apoptosis. 2006. 11:1583–1593.8. Wang TL, Chang H, Hung CR, Tseng YZ. Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc Res. 1998. 40:557–563.9. Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997. 80:1532–1535.10. Beauchamp P, Richard V, Tamion F, Lallemand F, Lebreton JP, Vaudry H, Daveau M, Thuillez C. Protective effects of preconditioning in cultured rat endothelial cells: effects on neutrophil adhesion and expression of ICAM-1 after anoxia and reoxygenation. Circulation. 1999. 100:541–546.11. Chen Z, Li T, Zhang B. Morphine postconditioning protects against reperfusion injury in the isolated rat hearts. J Surg Res. 2008. 145:287–294.12. Dershwitz M, Walsh JL, Morishige RJ, Connors PM, Rubsamen RM, Shafer SL, Rosow CE. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000. 93:619–628.13. Cao CM, Xia Q, Tu J, Chen M, Wu S, Wong TM. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J Pharmacol Exp Ther. 2004. 309:560–567.14. Yellon DM, Opie LH. Postconditioning for protection of the infarcting heart. Lancet. 2006. 367:456–458.15. Ela C, Barg J, Vogel Z, Hasin Y, Eilam Y. Distinct components of morphine effects on cardiac myocytes are mediated by the kappa and delta opioid receptors. J Mol Cell Cardiol. 1997. 29:711–720.16. Gearing AJ, Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993. 14:506–512.17. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992. 69:11–25.18. Rosen SD, Bertozzi CR. The selectins and their ligands. Curr Opin Cell Biol. 1994. 6:663–673.19. Shyu KG, Chang H, Lin CC, Kuan P. Circulating intercellular adhesion molecule-1 and E-selectin in patients with acute coronary syndrome. Chest. 1996. 109:1627–1630.20. Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA Jr. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987. 84:9238–9242.21. Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994. 12:141–179.22. Granger DN, Höllwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986. 548:47–63.23. Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009. 256:83–91.24. Ichikawa H, Flores S, Kvietys PR, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Circ Res. 1997. 81:922–931.25. Yoshida N, Granger DN, Anderson DC, Rothlein R, Lane C, Kvietys PR. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992. 262:H1891–H1898.26. Gross GJ. Role of opioids in acute and delayed preconditioning. J Mol Cell Cardiol. 2003. 35:709–718.27. Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004. 94:960–966.28. Fu LL, Xia Q, Shen YL, Wong TM. Involvement of endogenous opioids in cardioprotective effects of ischemic preconditioning in the isolated rat heart. Sheng Li Xue Bao. 1998. 50:603–610.29. Zhang Y, Chen ZW, Girwin M, Wong TM. Remifentanil mimics cardioprotective effect of ischemic preconditioning via protein kinase C activation in open chest of rats. Acta Pharmacol Sin. 2005. 26:546–550.30. Peart JN, Gross GJ. Cardioprotective effects of acute and chronic opioid treatment are mediated via different signaling pathways. Am J Physiol Heart Circ Physiol. 2006. 291:H1746–H1753.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphine Attenuates Endothelial Cell Adhesion Molecules Induced by the Supernatant of LPS-Stimulated Colon Cancer Cells
- Comparative Study on the Expression of ICAM-1 of Preimmune State Keratinocytes Induced by Trichophyton Antigen
- Expression of Intercellular Adhesion Molecule-l (ICAM-1) in Vascular Endothelium and Keratinocytes of Psoriatic Skin
- ICAM-1 and VCAM-1 on Human Umbilical Vein Endothelial Cells Infected by Hantaan Virus
- Increased Expression of ICAM-1 and VCAM-1 in Human Lupus Nephritis