J Korean Med Sci.
2009 Oct;24(5):945-950. 10.3346/jkms.2009.24.5.945.
Phase II Study of Combination Chemotherapy with Etoposide and Ifosfamide in Patients with Heavily Pretreated Recurrent or Persistent Epithelial Ovarian Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bgkim@skku.edu
- KMID: 1782020
- DOI: http://doi.org/10.3346/jkms.2009.24.5.945
Abstract
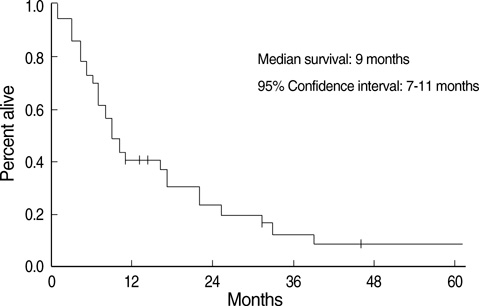
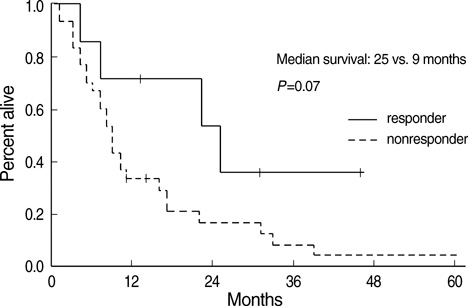
- The aim of this trial was to investigate the efficacy and toxicity of combination chemotherapy with etoposide and ifosfamide (ETI) in the management of heavily pretreated recurrent or persistent epithelial ovarian cancer (EOC). Patients with recurrent or persistent EOC who had measurable disease and at least two prior chemotherapy participating in this phase II trial were to receive etoposide at a dose of 100 mg/m2/day intravenously (IV) on days 1 to 3 in combination with ifosfamide 1 g/m2/day IV on days 1 to 5, every 21 days. Thirty-seven patients were treated; about 78% had previously received more than two separate regimens. The response rate (RR) was 18.9% and median duration of response was 7 months (range, 1-15). Treatment free interval prior to ETI (TFI) has significant correlation with RR rate (P=0.034). Patients (n=6) with TFI > or =6 months had 50% of RR, while patients (n=31) with TFI <6 months had 12.9%. Median survival was 9 months at a median follow-up of 9.2 months. Grade 3 or 4 toxicities included neutropenia in 20.1% of the 139 cycles of ETI, anemia in 7.2% and thrombocytopenia in 8.6%. The ETI produces relatively low toxicity and modest activity in heavily pretreated recurrent or persistent EOC. This is significant in patients with TFI > or =6 months.
Keyword
MeSH Terms
-
Adult
Aged
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Etoposide/administration & dosage/*therapeutic use
Female
Humans
Ifosfamide/administration & dosage/*therapeutic use
Middle Aged
Neoplasm Recurrence, Local/*drug therapy
Ovarian Neoplasms/*drug therapy/mortality
Survival Rate
Treatment Outcome
Figure
Cited by 1 articles
-
Retrospective study of combination chemotherapy with etoposide and ifosfamide in patients with heavily pretreated recurrent or persistent epithelial ovarian cancer
Wonkyo Shin, Hye-joo Lee, Seong J. Yang, E sun Paik, Hyun-jin Choi, Tae-Joong Kim, Chel Hun Choi, Jeong-Won Lee, Duk-Soo Bae, Byoung-Gie Kim
Obstet Gynecol Sci. 2018;61(3):352-358. doi: 10.5468/ogs.2018.61.3.352.
Reference
-
1. McMeekin DS, Tillmanns T, Chaudry T, Gold M, Johnson G, Walker J, Mannel R. Timing isn't everything: an analysis of when to start salvage chemotherapy in ovarian cancer. Gynecol Oncol. 2004. 95:157–164.
Article2. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.
Article3. Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, Alvarez RD, Kucera PR, Small JM. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2000. 18:106–115.
Article4. Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol. 1991. 9:389–393.
Article5. Bookman MA, Malmstrom H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ. Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol. 1998. 16:3345–3352.
Article6. Creemers GJ, Bolis G, Gore M, Scarfone G, Lacave AJ, Guastalla JP, Despax R, Favalli G, Kreinberg R, Van Belle S, Hudson I, Verweij J, Ten Bokkel Huinink WW. Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol. 1996. 14:3056–3061.
Article7. Shapiro JD, Millward MJ, Rischin D, Michael M, Walcher V, Francis PA, Toner GC. Activity of gemcitabine in patients with advanced ovarian cancer: responses seen following platinum and paclitaxel. Gynecol Oncol. 1996. 63:89–93.
Article8. Lund B, Hansen OP, Theilade K, Hansen M, Neijt JP. Phase II study of gemcitabine (2',2'-difluorodeoxycytidine) in previously treated ovarian cancer patients. J Natl Cancer Inst. 1994. 86:1530–1533.
Article9. Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia A, Burnett A, Greco FA, Morrow CP, Paradiso LJ, Liang LJ. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol. 1997. 15:987–993.
Article10. Bajetta E, Di Leo A, Biganzoli L, Mariani L, Cappuzzo F, Di Bartolomeo M, Zilembo N, Artale S, Magnani E, Celio L, Buzzoni R, Carnaghi C. Phase II study of vinorelbine in patients with pretreated advanced ovarian cancer: activity in platinum-resistant disease. J Clin Oncol. 1996. 14:2546–2551.
Article11. Hoskins PJ, Swenerton KD. Oral etoposide is active against platinum-resistant epithelial ovarian cancer. J Clin Oncol. 1994. 12:60–63.
Article12. Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998. 16:405–410.
Article13. Sutton GP, Blessing JA, Homesley HD, Berman ML, Malfetano J. Phase II trial of ifosfamide and mesna in advanced ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1989. 7:1672–1676.
Article14. Maskens AP, Armand JP, Lacave AJ, De Jager RL, Hansen HH, Wolff JP. Phase II clinical trial of VP-16-213 in ovarian cancer. Cancer Treat Rep. 1981. 65:329–330.15. Hillcoat BL, Campbell JJ, Pepperell R, Quinn MA, Bishop JF, Day A. Phase II trial of VP-16-213 in advanced ovarian carcinoma. Gynecol Oncol. 1985. 22:162–166.
Article16. Markman M, Hakes T, Reichman B, Lewis JL Jr, Rubin S, Jones W, Almadrones L, Pizzuto F, Hoskins W. Ifosfamide and mesna in previously treated advanced epithelial ovarian cancer: activity in platinum-resistant disease. J Clin Oncol. 1992. 10:243–248.
Article17. Lissoni AA, Fei F, Rossi R, Fruscio R, Villa A, Zani G. Ifosfamide in the treatment of malignant epithelial ovarian tumors. Oncology. 2003. 65:Suppl 2. 59–62.
Article18. Hainsworth JD, Greco FA. Etoposide: twenty years later. Ann Oncol. 1995. 6:325–341.
Article19. Trope CG, Kisic J, Vergote I. Prognostic factors in platinum-resistant ovarian carcinoma treated with ifosfamide-etoposide. Eur J Gynaecol Oncol. 2000. 21:255–259.20. Bruzzone M, Campora E, Merlini L, Giudici S, Bottero G, Iskra L, Donadio M, Ferrari I, Ragni N. Ifosfamide and etoposide salvage treatment in advanced ovarian cancer. J Chemother. 1991. 3:332–334.
Article21. Trope C, Kaern J, Vergote I, Vossli S. A phase II study of etoposide combined with ifosfamide as second-line therapy in cisplatin-resistant ovarian carcinomas. Cancer Chemother Pharmacol. 1990. 26:Suppl. S45–S47.22. Aravantinos G, Dimopoulos MA, Kosmidis P, Bafaloukos D, Papadimitriou C, Kiamouris C, Pavlidis N, Sikiotis K, Papakostas P, Skarlos DV. Ifosfamide plus oral etoposide salvage chemotherapy for platinum-resistant paclitaxel-pretreated ovarian cancer. Ann Oncol. 2000. 11:607–612.
Article23. Shaheen M, Stender MJ, McClean JW, Look KY, Einhorn LH. Phase II study of ifosfamide plus daily oral etoposide in previously treated ovarian cancer: a Hoosier Oncology Group (HOG) study. Am J Clin Oncol. 2004. 27:229–231.24. Vanhoefer U, Schleucher N, Klaassen U, Seeber S, Harstrick A. Ifosfamide-based drug combinations: preclinical evaluation of drug interactions and translation into the clinic. Semin Oncol. 2000. 27:8–13.25. Ozols RF. Treatment of recurrent ovarian cancer: increasing options--"recurrent" results. J Clin Oncol. 1997. 15:2177–2180.
Article26. Ovarian Cancer. V.I 2008. NCCN Clinical Practice Guidelines in Oncology. accessed on_. Available at http://www.nccn.org/professionals/physician_gls/PDF/ovarian.pdf.27. Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W, Torri V, Floriani I, Jayson G, Lamont A, Trope C. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003. 361:2099–2106.28. Rose PG. Gemcitabine reverses platinum resistance in platinum-resistant ovarian and peritoneal carcinoma. Int J Gynecol Cancer. 2005. 15:Suppl 1. 18–22.
Article29. ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson N, Spanczynski M, Bolis G, Malmstrom H, Coleman R, Fields SC, Heron JF. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997. 15:2183–2193.
Article30. Rothenberg ML, Liu PY, Wilczynski S, Nahhas WA, Winakur GL, Jiang CS, Moinpour CM, Lyons B, Weiss GR, Essell JH, Smith HO, Markman M, Alberts DS. Phase II trial of vinorelbine for relapsed ovarian cancer: a Southwest Oncology Group study. Gynecol Oncol. 2004. 95:506–512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retrospective study of combination chemotherapy with etoposide and ifosfamide in patients with heavily pretreated recurrent or persistent epithelial ovarian cancer
- Clinical Efficacy of Ifosfamide-Based Regimen in Refractory of Relapsed Ovarian Cancer
- Clinical Trial of IV Etoposide and Carboplatin and Cyclophosphamide Combination Chemotherapy Against Persistent or Recurrent Ovarian Cancer as 2nd or More Line Chemotherapy
- Fatal Ifosfamide-Induced Metabolic Encephalopathy in Patients with Recurrent Epithelial Ovarian Cancer: Report of Two Cases
- Ifosfamide and Etoposide in Relapsed Refractory Childhood Acute Lymphoblastic Leukemia