J Korean Med Sci.
2006 Apr;21(2):304-308. 10.3346/jkms.2006.21.2.304.
The Effect of Vero Cell Coculture on the Development of Mouse Embryos Exposed to Monoclonal Antibodies Specific for Mammalian Heat Shock Protein 60
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Inje University, Paik Hospital, Seoul, Korea. tttnii@hanmail.net
- KMID: 1781840
- DOI: http://doi.org/10.3346/jkms.2006.21.2.304
Abstract
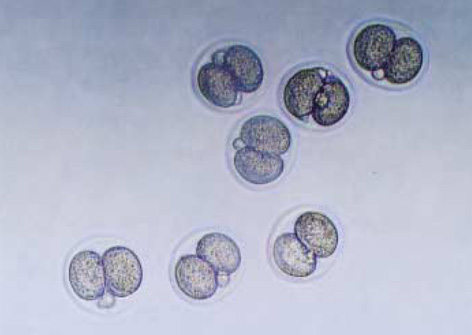
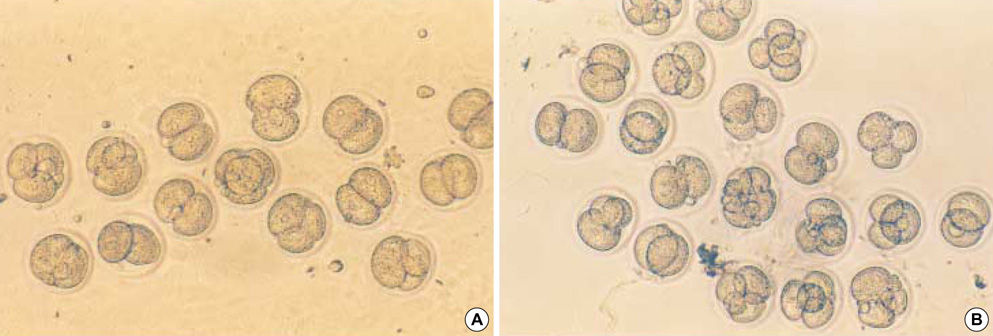
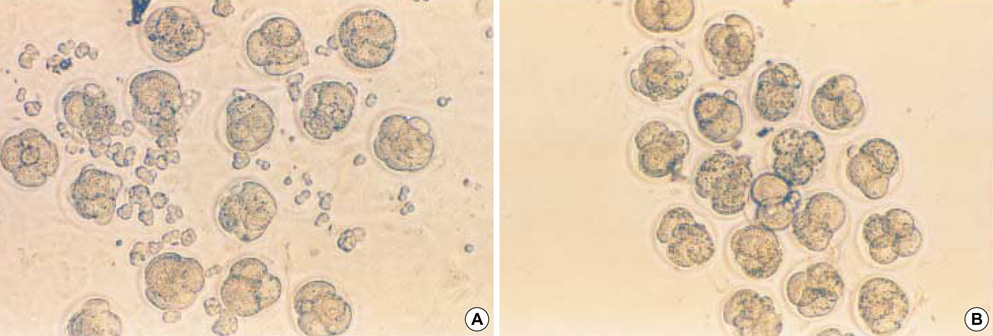
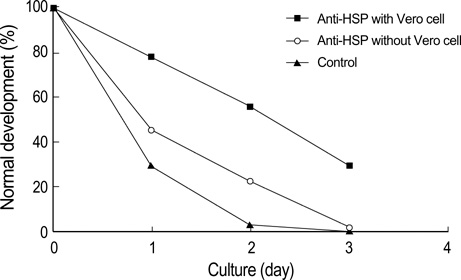
- Heat shock proteins (HSP) have been identified as an important factor of a very complex and highly conserved cellular defense mechanism to preserve cell survival under adverse environmental conditions. HSP 60 are immunodominant antigens of microbe such as Chlamydia trachomatis and have a potentiality to become a target antigen due to antigenic similarity between chlamydial and human HSP. This study was conducted to investigate the effects of Vero cell coculture to anti-HSP 60 on the early mouse embryo development in vitro. The 2-cell mouse embryos (ICR) were cultured and mouse embryo development was observed every 24 hr for 3 days. 45% and 22.1% of the embryos cultured in Ham's F-10 plus anti HSP 60 with Vero cells developed to the 4- to 8- cell stage (day 1) and morular stage (day 2) as compared with 29.2% and 2.7% of those cultured without Vero cells respectively. But at day 3, the beneficial effect of Vero cells was not noted. These findings suggest that Vero cells have some roles to overcome the detrimental effect of anti-HSP 60 to some degree. These results suggest that Vero cells coculture will promote reproductive outcome in patient previously sensitized to microbial (e.g. Chlamydia trachomatis) HSP 60.
Keyword
MeSH Terms
-
Vero Cells
Pregnancy
Mice, Inbred ICR
Mice
Male
Infertility, Female/etiology/immunology/therapy
Immunodominant Epitopes
Female
Embryonic Development/*immunology
Coculture Techniques
Chlamydia trachomatis/immunology/pathogenicity
Chaperonin 60/*immunology
Cercopithecus aethiops
Antigens, Bacterial
Antibodies, Monoclonal/*administration & dosage
Animals
Figure
Reference
-
1. Ritossa FA. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962. 18:571–573.2. Ellis RJ. Proteins as molecular chaperones. Nature. 1987. 328:378–379.
Article3. Lindquist S. The heat-shock response. Annu Rev Biochem. 1986. 55:1151–1191.
Article4. Soltys BJ, Gupta RS. Immunoelectron microscopic localization of the 60-kDa heat shock chaperonin protein (HSP 60) in mammalian cells. Exp Cell Res. 1996. 222:16–27.5. Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989. 9:2279–2283.
Article6. Ellis J. Stress proteins as molecular chaperones. 1996. New York: Marcel Decker;1–26.7. Witkin SS, Neuer A, Giraldo P, Jeremias J, Tolbert V, Korneeva IL, Kneissl D, Bongiovanni AM. Chlamydia trachomatis infection, immunity, and pregnancy outcome. Infect Dis Obstet Gynecol. 1997. 5:128–132.
Article8. Spandorfer SD, Neuer A, LaVerda D, Byrne G, Liu HC, Rosenwaks Z, Witkin SS. Previously undetected Chlamydia trachomatis infection, immunity to heat shock proteins and tubal occlusion in women undergoing in vitro fertilization. Hum Reprod. 1999. 14:60–64.9. Sakkas D, Trounsen AO, Kola I. In vivo cleavage rates and viability obtained for early cleavage mouse embryos in co-culture with oviductal cells. Reprod Fertil Dev. 1989. 1:127–136.10. Menezo YJ, Guerin JF, Czyba JC. Improvement of human early embryo development in vitro by coculture on monolayers of Vero cells. Biol Reprod. 1990. 42:301–306.11. Ouhibi N, Hamidi J, Guillaud J, Menezo Y. Co-culture of 1-cell mouse embryos on different cell supports. Hum Reprod. 1990. 5:737–743.
Article12. Bongso A, Gajra B, Lian NP, Wong PC, Soon-Chye N, Ratnam S. Establishment of human endometrial cell cultures. Hum Reprod. 1988. 3:705–713.
Article13. Menezo Y, Hazout A, Dumont M, Herbaut N, Nicollet B. Coculture of embryos on Vero cells and transfer of blastocysts in humans. Hum Reprod. 1992. 7:101–106.
Article14. Neuer A, Mele C, Liu HC, Rosenwaks Z, Witkin SS. Monoclonal antibodies to mammalian heat shock proteins impair mouse embryo development in vitro. Hum Reprod. 1998. 13:987–990.
Article15. Lee IH, Chung KN, Kim YB. Studies on the effects of monoclonal antibodies to mammalian heat shock protein 60 on mouse embryo development in vitro. Korean J Obstet Gynecol. 2003. 46:2216–2220.16. Moseley PL. Heat shock proteins and the inflammatory response. Ann NY Acad Sci. 1998. 856:206–213.
Article17. Shinnick TM. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Imminol. 1991. 167:145–160.
Article18. Beatty WL, Byrne GI, Morrison RP. Morphologic and antigenic characterization of interferon-γ mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993. 90:3998–4002.19. Legge M. Oocyte and zygote zona pellucida permeability to macromolecules. J Exp Zool. 1995. 271:145–150.
Article20. Alarcon-Segovia D, Ruiz-Arguelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cell. Immunol Today. 1996. 17:163–164.21. Goodeaux LL, Thibodeaux JK, Voelkel SA, Anzalone CA, Roussel JD, Cohen JC, Menezo YJ. Collection, co-culture, and transfer of Rhesus preimplantation embryos. Assit Reprod Technol Androl. 1990. 1:370–379.22. Van Blerkom J. Development of human embryos to the hatched blastocyst stage in the presence or absence of a monolayer of Vero cells. Hum Reprod. 1993. 8:1525–1539.
Article23. Gandolfi F, Brevini TA, Moor RM. Effect of oviduct environment on embryonic development. J Reprod Fertil Suppl. 1989. 38:107–115.24. Bongso A, Fong CY, Ng SC, Ratnam S. The search for improved in vitro systems should not be ignored: embryo coculture may be one of them. Hum Reprod. 1993. 8:1155–1162.25. Flood L, Shirley B. Reduction of embryotoxicity by protein in embryo culture media. Mol Reprod Dev. 1991. 30:226–231.
Article26. Fukui Y, McGowan LT, James RW, Pugh PA, Tervit HR. Factors affecting the in-vitro development to blastocysts of bovine oocytes matured and fertilized in-vitro. J Reprod Fertil. 1991. 92:125–131.27. Loutradis D, John D, Kiessling AA. Hypoxanthine causes a 2-cell block in random bred mouse embryos. Biol Reprod. 1987. 37:311–316.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Effects of Monoclonal Antibodies to Mammalian Heat Shock Protein 60 (HSP 60) on Mouse Embryo Development In Vitro
- The Effect of Coculture with Human Amniocyte on Growth and Development of Mouse 1-cell Stage Mouse Embryos
- The Effect of Coculture on Development of 2-Cell Stage Mouse Embryos
- The Effect of Coculture on In Vitro Fertilization of Oocytes and Development of Early Stage Embryos in Mice
- Comparative study on Development of Mouse Embryos in Conventional Medium versus Vero Cell Coculture