Korean J Lab Med.
2009 Feb;29(1):71-76. 10.3343/kjlm.2009.29.1.71.
Comparing Two Diagnostic Laboratory Tests for Several Microdeletions Causing Mental Retardation Syndromes: Multiplex Ligation-Dependent Amplification vs Fluorescent In Situ Hybridization
- Affiliations
-
- 1Greencross Reference Laboratory, Yongin, Korea. ehcho@mail.gcrl.co.kr
- KMID: 1781597
- DOI: http://doi.org/10.3343/kjlm.2009.29.1.71
Abstract
-
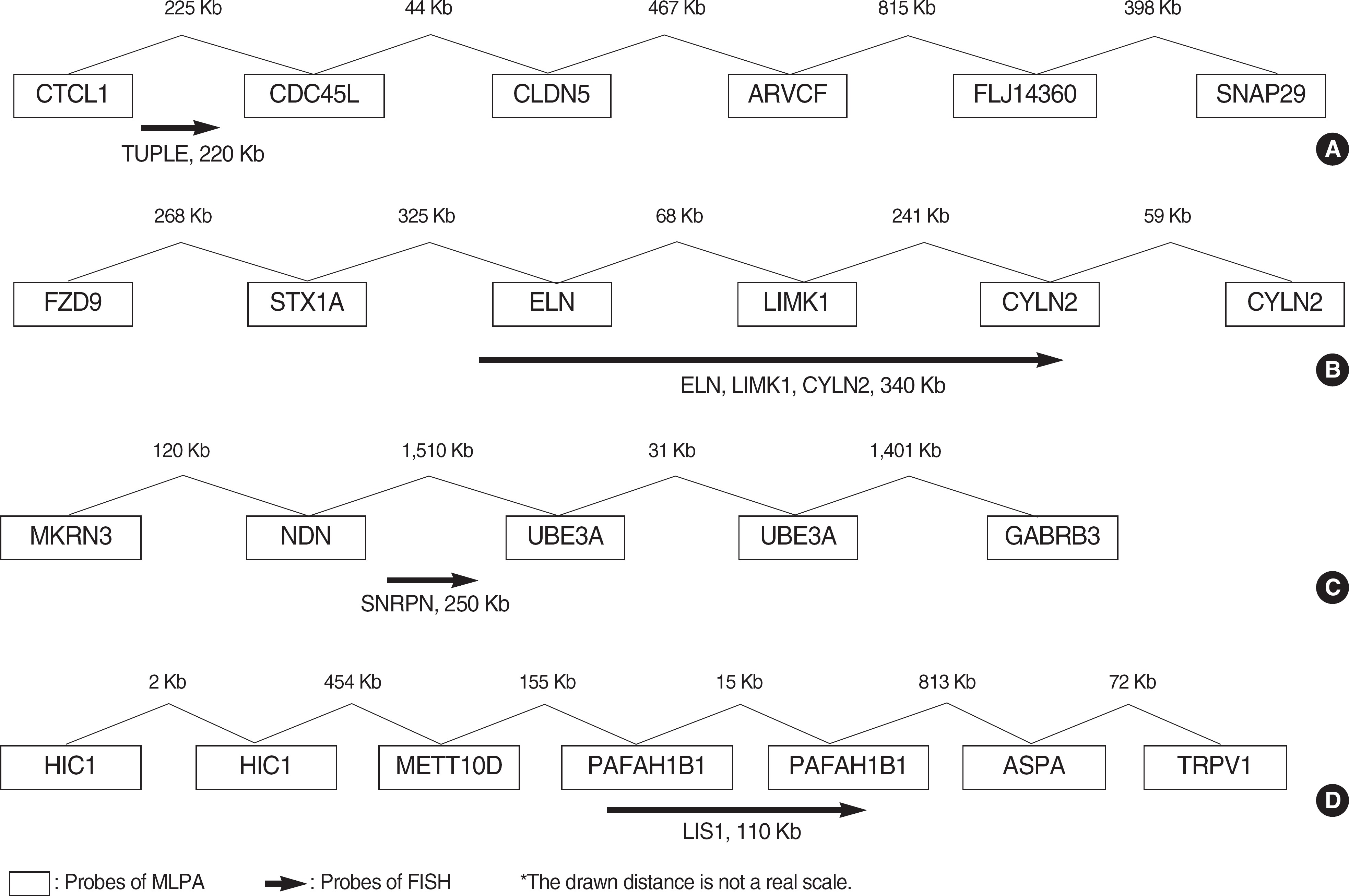
BACKGROUND: Microdeletion syndromes not detectable by conventional cytogenetic analysis have been reported to occur in approximately 5% of patients with unexplained mental retardation (MR). Therefore, it is essential to ensure that patients with MR are screened for these microdeletion syndromes. Mental retardation syndrome multiplex ligation-dependent probe amplification (MRS-MLPA) is a new technique for measuring sequence dosages that allows for the detection of copy number changes of several microdeletion syndromes (1p36 deletion syndrome, Williams syndrome, Smith-Magenis syndrome, Miller-Dieker syndrome, DiGeorge syndrome, Prader-Willi/Angelman syndrome, Alagille syndrome, Saethre-Chotzen syndrome, and Sotos syndrome) to be processed simultaneously, thus significantly reducing the amount of laboratory work.
METHODS
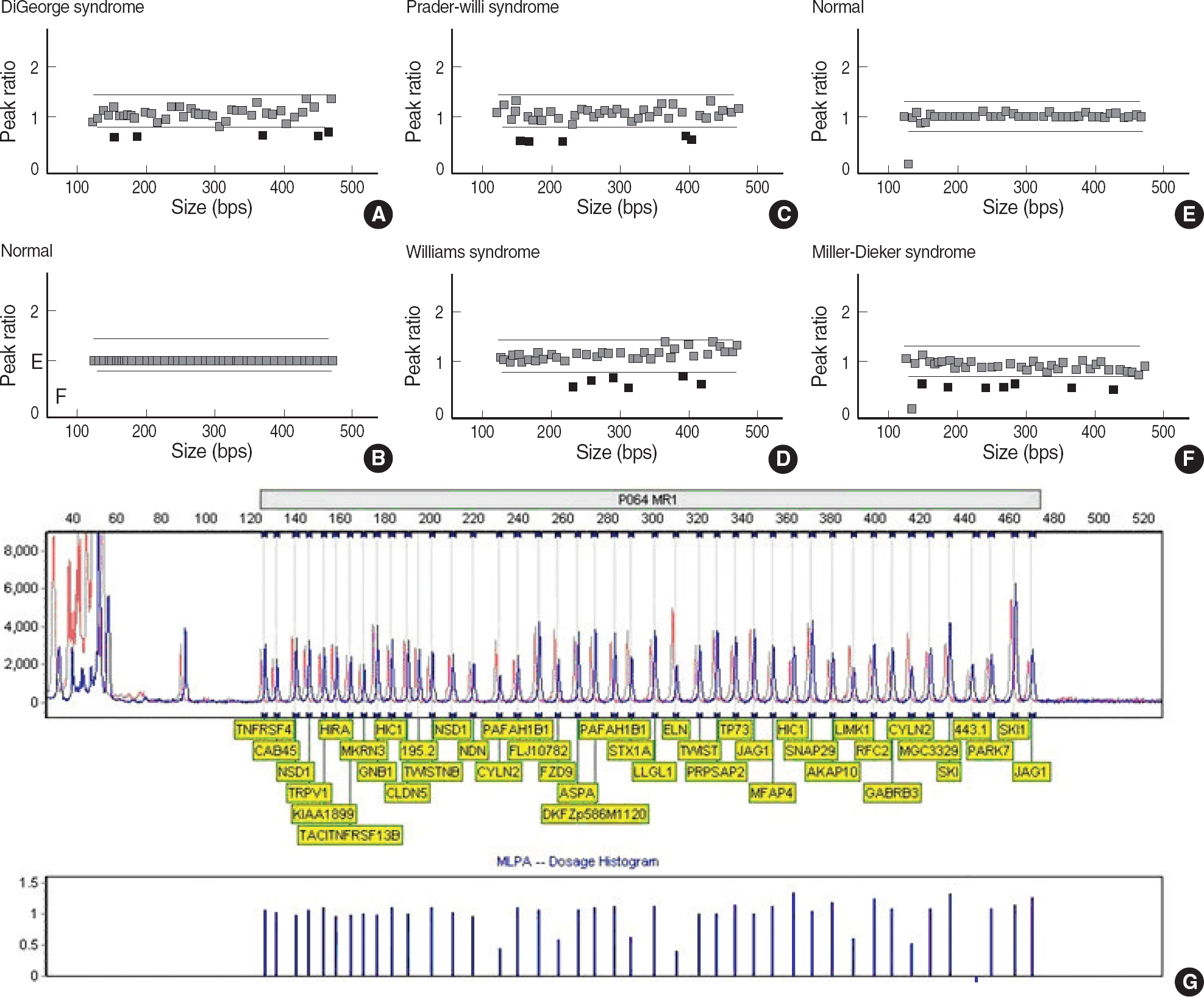
We assessed the performance of MLPA (MRC-Holland, The Netherlands) for the detection of microdeletion syndromes by comparing the results with those generated using FISH assays. MLPA analysis was carried out on 12 patients with microdeletion confirmed by FISH (three DiGeorge syndrome, four Williams syndrome, four Prader-Willi syndrome, and one Miller-Dieker syndrome).
RESULTS
The results of MLPA analysis showed a complete concordance with FISH in 12 patients with microdeletion syndromes.
CONCLUSIONS
On the basis of these results, we conclude that MLPA is an accurate, reliable, and cost-effective alternative to FISH in the screening for microdeletion syndromes.
MeSH Terms
-
*Chromosome Deletion
Classical Lissencephalies and Subcortical Band Heterotopias/genetics
DiGeorge Syndrome/genetics
Humans
In Situ Hybridization, Fluorescence/*methods
Laboratories, Hospital
Mental Retardation/*diagnosis/genetics
Nucleic Acid Amplification Techniques/*methods
Prader-Willi Syndrome/genetics
Williams Syndrome/genetics
Figure
Cited by 1 articles
-
Recombinant Chromosome 4 with Partial 4p Deletion and 4q Duplication Inherited from Paternal Pericentric Inversion
Se Jin Mun, Eun Hae Cho, Myoung-Jae Chey, Gyu-Hong Shim, Bo-Moon Shin, Rae-Kyung Lee, Ji-Kyung Ko, Soo Jin Yoo
Korean J Lab Med. 2010;30(1):89-92. doi: 10.3343/kjlm.2010.30.1.89.
Reference
-
1.Kirchhoff M., Bisgaard AM., Bryndorf T., Gerdes T. MLPA analysis for a panel of syndromes with mental retardation reveals imbalances in 5.8% of patients with mental retardation and dysmorphic features, including duplications of the Sotos syndrome and Williams-Beuren syndrome regions. Eur J Med Genet. 2007. 50:33–42.
Article2.Hunter AG. Outcome of the routine assessment of patients with mental retardation in a genetics clinic. Am J Med Genet. 2000. 90:60–8.
Article3.Sellner LN., Taylor GR. MLPA and MAPH: new techniques for detection of gene deletions. Hum Mutat. 2004. 23:413–9.
Article4.Rusu C., Sireteanu A., Puiu M., Skrypnyk C., Tomescu E., Csep K, et al. MLPA technique-principles and use in practice. Rev Med Chir Soc Med Nat Iasi. 2007. 111:1001–4.5.Rauch A., Hoyer J., Guth S., Zweier C., Kraus C., Becker C, et al. Diagnostic yield of various genetic approaches in patients with unexplained developmental delay or mental retardation. Am J Med Genet A. 2006. 140:2063–74.
Article6.Flint J., Knight S. The use of telomere probes to investigate sub-microscopic rearrangements associated with mental retardation. Curr Opin Genet Dev. 2003. 13:310–6.
Article7.Shaffer LG., Bejjani BA. A cytogeneticist's perspective on genomic microarrays. Hum Reprod Update. 2004. 10:221–6.
Article8.Wehner M., Mangold E., Sengteller M., Friedrichs N., Aretz S., Friedl W, et al. Hereditary nonpolyposis colorectal cancer: pitfalls in deletion screening in MSH2 and MLH1 genes. Eur J Hum Genet. 2005. 13:983–6.
Article9.Armour JA., Palla R., Zeeuwen PL., den Heijer M., Schalkwijk J., Hollox EJ. Accurate, high-throughput typing of copy number variation using paralogue ratios from dispersed repeats. Nucleic Acids Res. 2007. 35:e19.
Article10.White SJ., Vissers LE., Geurts van Kessel A., de Menezes RX., Kalay E., Lehesjoki AE, et al. Variation of CNV distribution in five different ethnic populations. Cytogenetic Genome Res. 2007. 118:19–30.
Article11.Janssen B., Hartmann C., Scholz V., Jauch A., Zschocke J. MLPA analysis for the detection of deletions, duplications and complex rearrangements in the dystrophin gene: potential and pitfalls. Neurogenetics. 2005. 6:29–35.
Article12.Redon R., Ishikawa S., Fitch KR., Feuk L., Perry GH., Andrews TD, et al. Global variation in copy number in the human genome. Nature. 2006. 444:444–54.
Article13.Shaikh TH., Kurahashi H., Saitta SC., O'Hare AM., Hu P., Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000. 9:489–501.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of Smith-Magenis Syndrome in a Patient with Mental Retardation and Sleep Disturbance Confirmed by Multiplex Ligation-dependent Probe Amplification
- Routine Chromosomal Microarray Analysis is Necessary in Korean Patients With Unexplained Developmental Delay/Mental Retardation/Autism Spectrum Disorder
- A Case of 18 Ring Chromosome
- Two Cases of alpha-thalassemia in Korean Children from Multicultural Family
- A Case of Wolf-Hirschhorn Syndrome with Long Term Survival Diagnosed by Fluorescent In-situ Hybridization (FISH)