Ann Lab Med.
2013 May;33(3):212-216. 10.3343/alm.2013.33.3.212.
A Case of Late-Onset Li-Fraumeni-like Syndrome with Unilateral Breast Cancer
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea. KAL1119@yuhs.ac
- 2Breast Cancer Center, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. GSJJOON@yuhs.ac
- KMID: 1781330
- DOI: http://doi.org/10.3343/alm.2013.33.3.212
Abstract
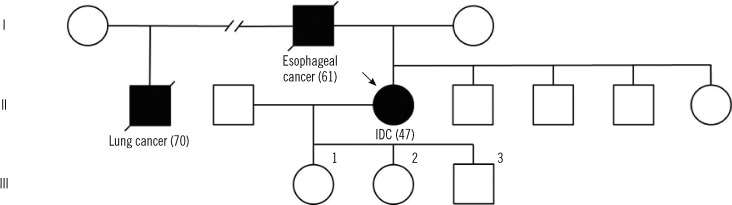
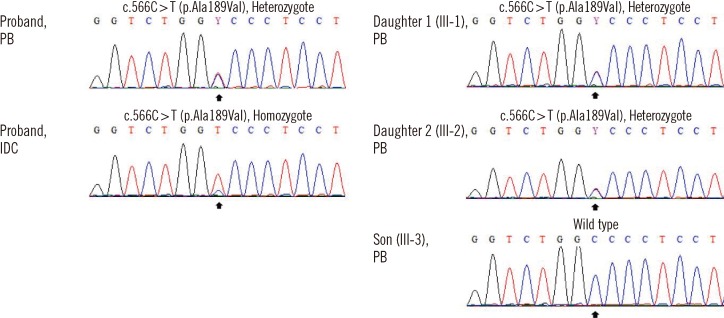
- Li-Fraumeni syndrome (LFS) is a rare, inherited syndrome associated with increased risk of various early-onset tumors. Since the introduction of classic LFS criteria, various criteria have been proposed to include patients with incomplete LFS features, which make up Li-Fraumeni-like syndromes (LFL). Germline missense mutations of TP53 are the primary cause of LFS and LFL. Mutations mostly reside in the DNA-binding domain of the gene and have a dominant-negative effect (DNE) over alternate wild-type alleles. Germline TP53 mutation c.566C>T results in the missense mutation GCC (Ala) to GTC (Val) at codon 189 (A189V) and has been reported in a case of multiple primary colon tumors. Herein we report a second case of the same mutation in a breast cancer patient, who has familial history of late-onset malignancies. Due to the relatively late onset of malignancies, neither case fulfils previously defined criteria for the syndrome. Mutational analysis for breast tissue in this patient showed a loss of heterozygosity. These clinical features may suggest a relatively weak DNE of A189V compared to other TP53 mutations, and in silico predictions and in vitro findings of the function of A189V mutant protein are conflicting. Considering the increased risk of malignancies and the therapeutic implications for patients who have a TP53 mutation, care must be taken when treating those who are suspected of possessing cancer-prone traits due to TP53 mutation, especially when there is a family history of late-onset cancer with low penetrance.
Keyword
MeSH Terms
-
Adolescent
Adult
Breast Neoplasms/complications/*diagnosis/therapy
Combined Modality Therapy
Exons
Female
Genotype
Heterozygote
Humans
Li-Fraumeni Syndrome/complications/*diagnosis/therapy
Middle Aged
Multimodal Imaging
Mutation, Missense
Pedigree
Sequence Analysis, DNA
Tumor Suppressor Protein p53/genetics
Young Adult
Tumor Suppressor Protein p53
Figure
Reference
-
1. Li FP, Fraumeni JF Jr. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969; 71:747–752. PMID: 5360287.2. Birch JM, Hartley AL, Tricker KJ, Prosser J, Condie A, Kelsey AM, et al. Prevalence and diversity of constitutional mutations in the p53 gene among 21 Li-Fraumeni families. Cancer Res. 1994; 54:1298–1304. PMID: 8118819.3. Eeles RA. Germline mutations in the TP53 gene. Cancer Surv. 1995; 25:101–124. PMID: 8718514.4. Chompret A, Brugières L, Ronsin M, Gardes M, Dessarps-Freichey F, Abel A, et al. P53 germline mutations in childhood cancers and cancer risk for carrier individuals. Br J Cancer. 2000; 82:1932–1937. PMID: 10864200.5. Chompret A, Abel A, Stoppa-Lyonnet D, Brugières L, Pagés S, Feunteun J, et al. Sensitivity and predictive value of criteria for p53 germline mutation screening. J Med Genet. 2001; 38:43–47. PMID: 11332399.6. Tinat J, Bougeard G, Baert-Desurmont S, Vasseur S, Martin C, Bouvignies E, et al. 2009 version of the Chompret criteria for Li Fraumeni syndrome. J Clin Oncol. 2009; 27:e108–e109. author reply e110. PMID: 19652052.
Article7. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000; 408:307–310. PMID: 11099028.
Article8. Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004; 23:6445–6470. PMID: 15322516.
Article9. Miyaki M, Iijima T, Ohue M, Kita Y, Hishima T, Kuroki T, et al. A novel case with germline p53 gene mutation having concurrent multiple primary colon tumours. Gut. 2003; 52:304–306. PMID: 12524418.
Article10. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410. PMID: 1757079.
Article11. Rubino C, de Vathaire F, Shamsaldin A, Labbe M, Lê MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer. 2003; 89:840–846. PMID: 12942115.
Article12. Heymann S, Delaloge S, Rahal A, Caron O, Frebourg T, Barreau L, et al. Radio-induced malignancies after breast cancer postoperative radiotherapy in patients with Li-Fraumeni syndrome. Radiat Oncol. 2010; 5:104. PMID: 21059199.
Article13. Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007; 26:2157–2165. PMID: 17401424.
Article14. Hainaut P, Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000; 77:81–137. PMID: 10549356.
Article15. Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007; 28:622–629. PMID: 17311302.16. Mathe E, Olivier M, Kato S, Ishioka C, Vaisman I, Hainaut P. Predicting the transactivation activity of p53 missense mutants using a four-body potential score derived from Delaunay tessellations. Hum Mutat. 2006; 27:163–172. PMID: 16395672.
Article17. Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc Natl Acad Sci U S A. 2003; 100:8424–8429. PMID: 12826609.
Article18. Collins JS, Schwartz CE. Detecting polymorphisms and mutations in candidate genes. Am J Hum Genet. 2002; 71:1251–1252. PMID: 12452182.
Article19. Dearth LR, Qian H, Wang T, Baroni TE, Zeng J, Chen SW, et al. Inactive full-length p53 mutants lacking dominant wild-type p53 inhibition highlight loss of heterozygosity as an important aspect of p53 status in human cancers. Carcinogenesis. 2007; 28:289–298. PMID: 16861262.
Article20. Olivier M, Goldgar DE, Sodha N, Ohgaki H, Kleihues P, Hainaut P, et al. Li-Fraumeni and related syndromes: correlation between tumor type, family structure, and TP53 genotype. Cancer Res. 2003; 63:6643–6650. PMID: 14583457.21. Li FP, Fraumeni JF Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988; 48:5358–5362. PMID: 3409256.22. Garber JE, Goldstein AM, Kantor AF, Dreyfus MG, Fraumeni JF Jr, Li FP. Follow-up study of twenty-four families with Li-Fraumeni syndrome. Cancer Res. 1991; 51:6094–6097. PMID: 1933872.23. Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009; 27:1250–1256. PMID: 19204208.
Article24. Melhem-Bertrandt A, Bojadzieva J, Ready KJ, Obeid E, Liu DD, Gutierrez-Barrera AM, et al. Early onset HER2-positive breast cancer is associated with germline TP53 mutations. Cancer. 2012; 118:908–913. PMID: 21761402.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Breast Cancer after Radiation Therapy in a Patient with Li-Fraumeni Syndrome: A Case Report
- The first documentation of Li-Fraumeni syndrome in Korea
- Genetic Counseling Can Influence the Course of a Suspected Familial Cancer Syndrome Patient: From a Case of Li-Fraumeni Like Syndrome with a Germline Mutation in the TP53 Gene
- Family of Li-Fraumeni Syndrome with a Germline Mutation in the p53 Gene
- Osteosarcoma with Adenocarcinoma of Lung in Li-Fraumeni Syndrome: A Case Report