Ann Lab Med.
2013 Jan;33(1):8-13. 10.3343/alm.2013.33.1.8.
Activated Protein C Anticoagulant System Dysfunction and Thrombophilia in Asia
- Affiliations
-
- 1Department of Clinical Chemistry, Faculty of Pharmaceutical Sciences, Natagaki International University, Nagasaki, Japan. hamasaki-nao@niu.ac.jp
- 2Department of Nutrition Sciences, Nakamura Gakuen University, Fukuoka, Japan.
- KMID: 1781292
- DOI: http://doi.org/10.3343/alm.2013.33.1.8
Abstract
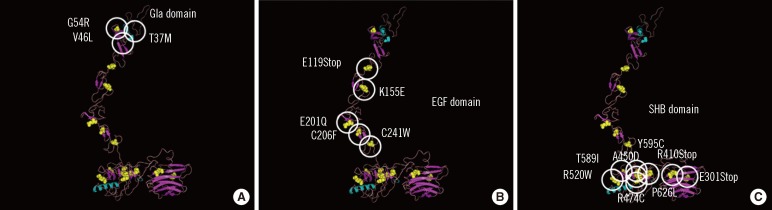
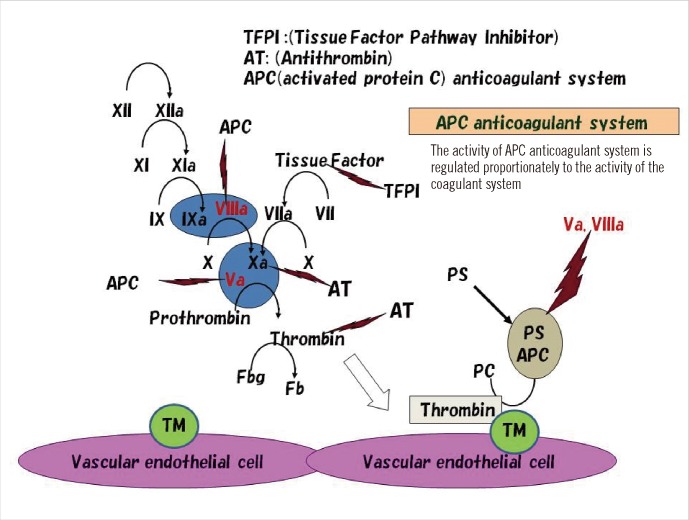
- Thrombophilia that is common among Caucasians is caused by genetic polymorphisms of coagulation factor V Leiden (R506Q) and prothrombin G20210A. Unlike that in Caucasians, thrombophilia that is common in the Japanese and Chinese involve dysfunction of the activated protein C (APC) anticoagulant system caused by abnormal protein S and protein C molecules. Approximately 50% of Japanese and Chinese individuals who develop venous thrombosis have reduced activities of protein S. The abnormal sites causing the protein S molecule abnormalities are distributed throughout the protein S gene, PROS1. One of the most common abnormalities is protein S Tokushima (K155E), which accounts for about 30% of the protein S molecule abnormalities in the Japanese. Whether APC dysfunction occurs in other Asian countries is an important aspect of mapping thrombophilia among Asians. International surveys using an accurate assay system are needed to determine this.
Keyword
MeSH Terms
Figure
Reference
-
1. Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, et al. Inherited thrombophilia: Part 1. Thromb Haemost. 1996; 76:651–662. PMID: 8950768.
Article2. Lane DA, Mannucci PM, Bauer KA, Bertina RM, Bochkov NP, Boulyjenkov V, et al. Inherited thrombophilia: Part 2. Thromb Haemost. 1996; 76:824–834. PMID: 8971998.
Article3. Dahlbäck B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci USA. 1993; 90:1004–1008. PMID: 8430067.4. Koster T, Rosendaal FR, de Ronde H, Briët E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden Thrombophilia Study. Lancet. 1993; 342:1503–1506. PMID: 7902898.
Article5. Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994; 369:64–67. PMID: 8164741.
Article6. Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the gene coding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995; 332:912–917. PMID: 7877648.
Article7. Rosendaal FR. Risk factors for venous thrombosis: prevalence, risk, and interaction. Semin Hematol. 1997; 34:171–187. PMID: 9241704.8. Castoldi E, Rosing J. APC resistance: biological basis and acquired influences. J Thromb Haemost. 2010; 8:445–453. PMID: 20002539.
Article9. Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996; 88:3698–3703. PMID: 8916933.
Article10. Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001; 109:369–384. PMID: 11702218.
Article11. Bounameaux H, Rosendaal FR. Venous thromboembolism: Why does ethnicity matter? Circulation. 2011; 123:2189–2191. PMID: 21555708.12. Rees DC, Cox M, Clegg JB. World distribution of factor V Leiden. Lancet. 1995; 346:1133–1134. PMID: 7475606.
Article13. Seki T, Okayama H, Kumagai T, Kumasaka N, Sakuma M, Isoyama S, et al. Arg506Gln mutation of the coagulation factor V gene not detected in Japanese pulmonary thromboembolism. Heart Vessels. 1998; 13:195–198. PMID: 10442401.
Article14. Isshiki I, Murata M, Watanabe R, Matsubara Y, Kawano K, Aoki N, et al. Frequencies of prothrombin 20210 G A mutation may be different among races--studies on Japanese populations with various forms of thrombotic disorders and healthy subjects. Blood Coagul Fibrinolysis. 1998; 9:105–106. PMID: 9607126.15. Shen MC, Lin JS, Tsay W. High prevalence of antithrombin III, protein C and protein S deficiency, but no factor V Leiden mutation in venous thrombophilic Chinese patients in Taiwan. Thromb Res. 1997; 87:377–385. PMID: 9271815.
Article16. Kim YW, Yoon KY, Park S, Shim YS, Cho HI, Park SS. Absence of factor V Leiden mutation in Koreans. Thromb Res. 1997; 86:181–182. PMID: 9175239.17. Tang L, Guo T, Yang R, Mei H, Wang H, Lu X, et al. Genetic background analysis of protein C deficiency demonstrates a recurrent mutation associated with venous thrombosis in Chinese population. PLoS One. 2012; 7:e35773. PMID: 22545135.
Article18. Kinoshita S, Iida H, Inoue S, Watanabe K, Kurihara M, Wada Y, et al. Protein S and protein C gene mutations in Japanese deep vein thrombosis patients. Clin Biochem. 2005; 38:908–915. PMID: 15978566.
Article19. Liu HW, Kwong YL, Bourke C, Lam CK, Lie AK, Wei D, et al. High incidence of thrombophilia detected in Chinese patients with venous thrombosis. Thromb Haemost. 1994; 71:416–419. PMID: 8052955.
Article20. Tsuda H, Hattori S, Tanabe S, Iida H, Nakahara M, Nishioka S, et al. Screening for aetiology of thrombophilia: a high prevalence of protein S abnormality. Ann Clin Biochem. 1999; 36:423–432. PMID: 10456203.
Article21. Miyata T, Hamasaki N, Wada H, Kojima T. More on: racial differences in venous thromboembolism. J Thromb Haemost. 2012; 10:319–320. PMID: 22141429.
Article22. Trujillo-Santos AJ, Jiménez-Puente A, Perea-Milla E. Association between long travel and venous thromboembolic disease: a systematic review and meta-analysis of case-control studies. Ann Hematol. 2008; 87:79–86. PMID: 17899081.
Article23. House of Commons Health Committee. The prevention of venous thromboembolism in hospitalised patients (Second report of session 2004-2005). Updated on Mar 2005. http://www.publications.parliament.uk/pa/cm200405/cmselect/cmhealth/99/99.pdf.24. Bhatia V, AroraP , Parida AK, Mittal A, Pandey AK, Kaul U. Air travel and pulmonary embolism: "economy class syndrome". J Assoc Physicians India. 2009; 57:412–414. PMID: 19634292.25. Ueda S, Hanzawa K, Shibata M, Suzuki S. High prevalence of deep vein thrombosis in tsunami-flooded shelters established after the great East-Japan earthquake. Tohoku J Exp Med. 2012; 227:199–202. PMID: 22728376.
Article26. Shigekiyo T, Uno Y, Kawauchi S, Saito S, Hondo H, Nishioka J, et al. Protein S Tokushima: an abnormal protein S found in a Japanese family with thrombosis. Thromb Haemost. 1993; 70:244–246. PMID: 8236127.
Article27. Yamazaki Y, Sugiura I, Matsushita T, Kojima T, Kagami K, Takamatsu J, et al. A phenotypically neutral dimorphism of protein S: the substitution of Lys155 by Glu in the second EGF domain predicted by an A to G base exchange in the gene. Thromb Res. 1993; 70:395–403. PMID: 8378895.
Article28. Hayashi T, Nishioka J, Shigekiyo T, Saito S, Suzuki K. Protein S Tokushima: abnormal molecule with a substitution of Glu for Lys-155 in the second epidermal growth factor-like domain of protein S. Blood. 1994; 83:683–690. PMID: 8298131.
Article29. Tsuda H, Urata M, Tsuda T, Wakiyama M, Iida H, Nakahara M, et al. Four missense mutations identified in the protein S gene of thrombosis patients with protein S deficiency: effects on secretion and anticoagulant activity of protein S. Thromb Res. 2002; 105:233–239. PMID: 11927129.30. Kimura R, Honda S, Kawasaki T, Tsuji H, Madoiwa S, Sakata Y, et al. Protein S-K196E mutation as a genetic risk factor for deep vein thrombosis in Japanese patients. Blood. 2006; 107:1737–1738. PMID: 16461766.
Article31. Ikejiri M, Wada H, Sakamoto Y, Ito N, Nishioka J, Nakatani K, et al. The association of protein S Tokushima-K196E with a risk of deep vein thrombosis. Int J Hematol. 2010; 92:302–305. PMID: 20811787.
Article32. Hamasaki N. Japanese thrombophilia: Protein S/Protein C anomaly as the major risk factor for Japanese thrombophilia. Nihon Kessen Shiketsu Gakkai shi. 2006; 17:136–143. (in Japanese).33. Tatewaki H, Iida H, Nakahara M, Tsuda H, Kinoshita S, Kanaji T, et al. A novel splice acceptor site mutation which produces multiple splicing abnormalities resulting in protein S deficiency type I. Thromb Haemost. 1999; 82:65–71. PMID: 10456456.
Article34. Nakahara M, Iida H, Urata M, Fujise M, Wakiyama M, Kinoshita S, et al. A novel splice acceptor site mutation of protein S gene in affected individuals with type I protein S deficiency: allelic exclusion of the mutant gene. Thromb Res. 2001; 101:387–393. PMID: 11297755.
Article35. Iida H, Nakahara M, Komori K, Fujise M, Wakiyama M, Urata M, et al. Failure in the detection of aberrant mRNA from the heterozygotic splice site mutant allele for protein S in a patient with protein S deficiency. Thromb Res. 2001; 102:187–196. PMID: 11369411.
Article36. Tsuda H, Tokunaga F, Nagamitsu H, Koide T. Characterization of endoplasmic reticulum-associated degradation of a protein S mutant identified in a family of quantitative protein S deficiency. Thromb Res. 2006; 117:323–331. PMID: 15893367.
Article37. Yamazaki T, Hamaguchi M, Katsumi A, Kagami K, Kojima T, Takamatsu J, et al. A quantitative protein S deficiency associated with a novel nonsense mutation and markedly reduced levels of mutated mRNA. Thromb Haemost. 1995; 74:590–595. PMID: 8584989.
Article38. Yamazaki T, Katsumi A, Kagami K, Okamoto Y, Sugiura I, Hamaguchi M, et al. Molecular basis of a hereditary type I protein S deficiency caused by a substitution of Cys for Arg474. Blood. 1996; 87:4643–4650. PMID: 8639833.
Article39. Okamoto Y, Yamazaki T, Katsumi A, Kojima T, Takamatsu J, Nishida M, et al. A novel nonsense mutation associated with an exon skipping in a patient with hereditary protein S deficiency type I. Thromb Haemost. 1996; 75:877–882. PMID: 8822579.
Article40. Yamazaki T, Katsumi A, Okamoto Y, Takafuta T, Tsuzuki S, Kagami K, et al. Two distinct novel splice site mutations in a compound heterozygous patient with protein S deficiency. Thromb Haemost. 1997; 77:14–20. PMID: 9031442.
Article41. Fujimura H, Kambayashi J, Kato H, Sakon M, Kawasaki T, Ariyoshi H, et al. Three novel missense mutations in unrelated Japanese patients with type I and type II protein S deficiency and venous thrombosis. Thromb Res. 1998; 89:151–160. PMID: 9651142.
Article42. Iwaki T, Mastushita T, Kobayashi T, Yamamoto Y, Nomura Y, Kagami K, et al. DNA sequence analysis of protein S deficiency--identification of four point mutations in twelve Japanese subjects. Semin Thromb Hemost. 2001; 27:155–160. PMID: 11372770.
Article43. Yamazaki T, Saito H, Dahlbäck B. Rapid intracellular degradation of a truncated mutant protein S (Q522X). Thromb Haemost. 2002; 87:171–172. PMID: 11848449.
Article44. Hirose M, Kimura F, Wang HQ, Takebayashi K, Kobayashi M, Nakanishi K, et al. Protein S gene mutation in a young woman with type III protein S deficiency and venous thrombosis during pregnancy. J Thromb Thrombolysis. 2002; 13:85–88. PMID: 12101385.45. Okada H, Takagi A, Murate T, Adachi T, Yamamoto K, Matsushita T, et al. Identification of protein Sα gene mutations including four novel mutations in eight unrelated patients with protein S deficiency. Br J Haematol. 2004; 126:219–225. PMID: 15238143.
Article46. Okada H, Yamazaki T, Takagi A, Murate T, Yamamoto K, Takamatsu J, et al. In vitro characterization of missense mutations associated with quantitative protein S deficiency. J Thromb Haemost. 2006; 4:2003–2009. PMID: 16961607.47. Mizukami K, Nakabayashi T, Naitoh S, Takeda M, Tarumi T, Mizoguchi I, et al. One novel and one recurrent mutation in the PROS1 gene cause type I protein S deficiency in patients with pulmonary embolism associated with deep vein thrombosis. Am J Hematol. 2006; 81:787–797. PMID: 16868938.
Article48. Sanda N, Fujimori Y, Kashiwagi T, Takagi A, Murate T, Mizutani E, et al. An Sp1 binding site mutation of the PROS1 promoter in a patient with protein S deficiency. Br J Haematol. 2007; 138:663–665. PMID: 17596203.
Article49. Hamasaki N, Kanaji T. Tanaka K, Davie EW, editors. Clinical role of protein S deficiency in Asian population. Recent advances in thrombosis and hemostasis. 2008. Japan: Springer;p. 597–613.
Article50. Bonthron D. The human von Willebrand factor gene. Structure of the 5' region. Eur J Biochem. 1988; 171:51–57. PMID: 2828057.
Article51. Mancuso DJ, Tuley EA, Westfield LA, Lester-Mancuso TL, Le Beau MM, Sorace JM, et al. Human von Willebrand factor gene and pseudogene: structural analysis and differentiation by polymerase chain reaction. Biochemistry. 1991; 30:253–269. PMID: 1988024.
Article52. Johansson AM, Hillarp A, Säll T, Zöller B, Dahlbäck B, Halldén C. Large deletions of the PROS1 gene in a large fraction of mutation-negative patients with protein S deficiency. Thromb Haemost. 2005; 94:951–957. PMID: 16363235.
Article53. Lind-Halldén C, Dahlen A, Hillarp A, Zöller B, Dahlbäck B, Halldén C. Small and large PROS1 deletions but no other types of rearrangements detected in patients with protein S deficiency. Thromb Haemost. 2012; 108:94–100. PMID: 22627709.54. Hamasaki N. Unmasking Asian thrombophilia: is APC dysfunction the real culprit? J Thromb Haemost. 2012; 10:2016–2018. PMID: 22905992.
Article55. Kimura R, Sakata T, Kokubo Y, Okamoto A, Tomoike H, Miyata T. Plasma protein S activity correlates with protein S genotype but is not sensitive to identify K196E mutant carriers. J Thromb Haemost. 2006; 4:2010–2013. PMID: 16961608.
Article56. Tsuda T, Jin X, Tsuda H, Ieko M, Morishita E, Adachi T, et al. New quantitative total protein S-assay system for diagnosing of protein S type II deficiency: clinical application of the screening system for protein S type II deficiency. Blood Coagul Fibrinolysis. 2012; 23:56–63. PMID: 22157257.57. Suzuki A, Sanda N, Miyawaki Y, Fujimori Y, Yamada T, Takagi A, et al. Down-regulation of PROS1 gene expression by 17β-estradiol via estrogen receptor α (ERα)-Sp1 interaction recruiting receptor-interacting protein 140 and the corepressor-HDAC3 complex. J Biol Chem. 2010; 285:13444–13453. PMID: 20200160.58. Drakenberg T, Ghasriani H, Thulin E, Thämlitz AM, Muranyi A, Annila A, et al. Solution structure of the Ca2+-Binding EGF3-4 pair from vitamin K-dependent protein S: identification of an unusual fold in EGF3. Biochemistry. 2005; 44:8782–8789. PMID: 15952784.
Article59. Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003; 31:3381–3385. PMID: 12824332.
Article60. Huang M, Furie BC, Furie B. Crystal structure of the calcium-stabilized human factor IX Gla domain bound to a conformation-specific anti-factor IX antibody. J Biol Chem. 2004; 279:14338–14346. PMID: 14722079.
Article61. Groebke Zbinden K, Banner DW, Ackermann J, D'Arcy A, Kirchhofer D, Ji YH, et al. Design of selective phenylglycine amide tissue factor/factor VIIa inhibitors. Bioorg Med Chem Lett. 2005; 15:817–822. PMID: 15664864.
Article62. Sasaki T, Knyazev PG, Cheburkin Y, Göhring W, Tisi D, Ullrich A, et al. Crystal structure of a C-terminal fragment of growth arrest-specific protein Gas6. Receptor tyrosine kinase activation by laminin G-like domains. J Biol Chem. 2002; 277:44164–44170. PMID: 12218057.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Renal Infarction with Protein S Deficiency
- Recurrent Deep Vein Thrombosis due to Thrombophilia
- A Case of Behcet's Disease Associated with Protein S Deficiency
- Hematologic Risk Factors in Young-Aged Retinal Vein Occlusion
- First Korean case of factor V Leiden mutation in pregnant woman with a history of recurrent pregnancy loss