Yonsei Med J.
2010 Jan;51(1):82-87. 10.3349/ymj.2010.51.1.82.
Effect of Sildenafil on Neuropathic Pain and Hemodynamics in Rats
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University Medical School, Gwangju, Korea. mhyoon@chonnam.ac.kr
- 2The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Korea.
- KMID: 1779610
- DOI: http://doi.org/10.3349/ymj.2010.51.1.82
Abstract
- PURPOSE
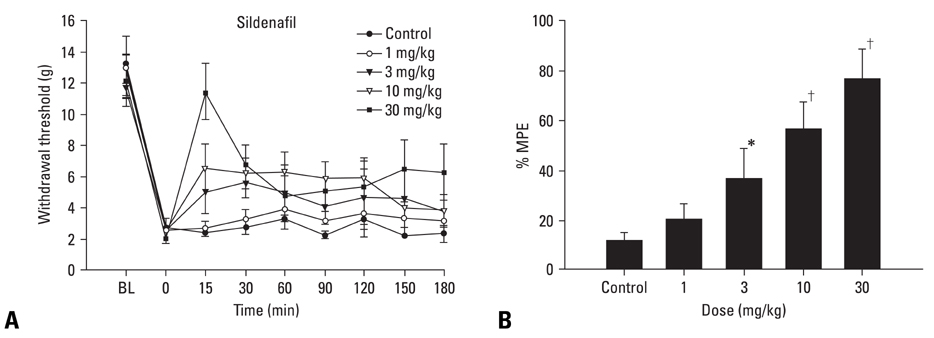
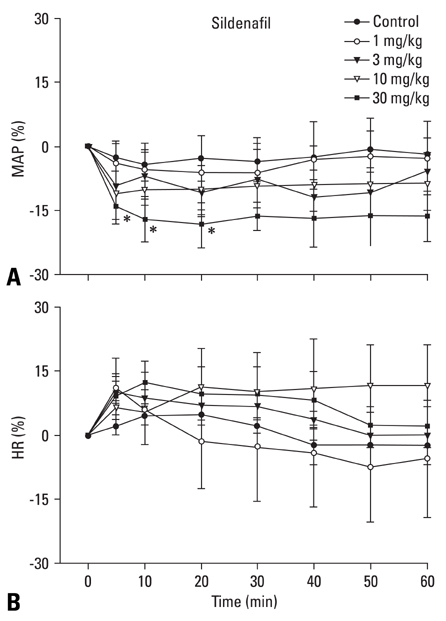
The inhibition of phosphodiesterase 5 produces an antinociception through the increase of cyclic guanosine monophosphate (cGMP), and increasing cGMP levels enhance the release of gamma-aminobutyric acid (GABA). Furthermore, this phosphodiesterase 5 plays a pivotal role in the regulation of the vasodilatation associated to cGMP. In this work, we examined the contribution of GABA receptors to the effect of sildenafil, a phosphodiesterase 5 inhibitor, in a neuropathic pain rat, and assessed the hemodynamic effect of sildenafil in normal rats. MATERIALS AND METHODS: Neuropathic pain was induced by ligation of L5/6 spinal nerves in Sprague-Dawley male rats. After observing the effect of intravenous sildenafil on neuropathic pain, GABAA receptor antagonist (bicuculline) and GABAB receptor antagonist (saclofen) were administered prior to delivery of sildenafil to determine the role of GABA receptors in the activity of sildenafil. For hemodynamic measurements, catheters were inserted into the tail artery. Mean arterial pressure (MAP) and heart rate (HR) were measured over 60 min following administration of sildenafil. RESULTS: Intravenous sildenafil dose-dependently increased the withdrawal threshold to the von Frey filament application in the ligated paw. Intravenous bicuculline and saclofen reversed the antinociception of sildenafil. Intravenous sildenafil increased the magnitude of MAP reduction at the maximal dosage, but it did not affect HR response. CONCLUSION: These results suggest that sildenafil is active in causing neuropathic pain. Both GABAA and GABAB receptors are involved in the antinociceptive effect of sildenafil. Additionally, intravenous sildenafil reduces MAP without affecting HR.
Keyword
MeSH Terms
-
Animals
Baclofen/analogs & derivatives/pharmacology
Bicuculline/pharmacology
Blood Pressure/drug effects
Dose-Response Relationship, Drug
Heart Rate/drug effects
Hemodynamics/drug effects
Male
Neuralgia/*drug therapy
Pain Threshold/drug effects
Phosphodiesterase Inhibitors/*therapeutic use
Piperazines/*therapeutic use
Purines/therapeutic use
Rats
Rats, Sprague-Dawley
Receptors, GABA-A/antagonists & inhibitors/physiology
Receptors, GABA-B/antagonists & inhibitors/physiology
Sulfones/*therapeutic use
Figure
Reference
-
1. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007. 132:237–251.
Article2. Finnerup NB, Sindrup SH, Jensen TS. Chronic neuropathic pain: mechanisms, drug targets and measurement. Fundam Clin Pharmacol. 2007. 21:129–136.
Article3. Wallace JM. Update on pharmacotherapy guidelines for treatment of neuropathic pain. Curr Pain Headache Rep. 2007. 11:208–214.
Article4. Chizh BA, Göhring M, Tröster A, Quartey GK, Schmelz M, Koppert W. Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth. 2007. 98:246–254.
Article5. Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005. 132:193–201.
Article6. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.
Article7. Pyne NJ, Arshavsky V, Lochhead A. cGMP signal termination. Biochem Soc Trans. 1996. 24:1019–1022.
Article8. Ferreira SH, Nakamura M. I-Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979. 18:179–190.
Article9. Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001. 897:9–19.10. Araiza-Saldaña CI, Reyes-García G, Bermúdez-Ocaña DY, Pérez-Severiano F, Granados-Soto V. Effect of diabetes on the mechanisms of intrathecal antinociception of sildenafil in rats. Eur J Pharmacol. 2005. 527:60–70.
Article11. Asomoza-Espinosa R, Alonso-López R, Mixcoatl-Zecuatl T, Aguirre-Bañuelos P, Torres-López JE, Granados-Soto V. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol. 2001. 418:195–200.
Article12. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001. 909:170–178.
Article13. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003. 67:150–156.
Article14. Mixcoatl-Zecuatl T, Aguirre-Bañuelos P, Granados-Soto V. Sildenafil produces antinociception and increases morphine antinociception in the formalin test. Eur J Pharmacol. 2000. 400:81–87.
Article15. Saransaari P, Oja SS. Characteristics of GABA release induced by free radicals in mouse hippocampal slices. Neurochem Res. 2008. 33:384–393.16. Kim WM, Yoon MH, Lee HG, Han YG, Kim YO, Huang LJ, et al. GABAB receptor modulation on the antinociception of intrathecal sildenafil in the rat formalin test. Korean J Pain. 2007. 20:106–110.
Article17. Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999. 83:3C–12C.18. Matsumoto T, Kobayashi T, Kamata K. Phosphodiesterases in the vascular system. J Smooth Muscle Res. 2003. 39:67–86.
Article19. Santos-Silva AJ, Cairrão E, Morgado M, Alvarez E, Verde I. PDE4 and PDE5 regulate cyclic nucleotides relaxing effects in human umbilical arteries. Eur J Pharmacol. 2008. 582:102–109.
Article20. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.
Article21. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994. 53:55–63.
Article22. Blandizzi C, Bernardini MC, Natale G, Martinotti E, Del Tacca M. Peripheral 2-hydroxy-saclofen-sensitive GABA-B receptors mediate both vagal-dependent and vagal-independent acid secretory responses in rats. J Auton Pharmacol. 1992. 12:149–156.
Article23. Dogrul A, Ossipov MH, Lai J, Malan TP Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000. 292:115–118.24. Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007. 132:237–251.25. Dray A. Neuropathic pain: emerging treatments. Br J Anaesth. 2008. 101:48–58.
Article26. Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006. 7:281–289.
Article27. Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990. 11:150–155.
Article28. Uckert S, Hedlund P, Andersson KE, Truss MC, Jonas U, Stief CG. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future. Eur Urol. 2006. 50:1194–1207.
Article29. Freund TF, Buzsáki G. Alterations in excitatory and GABAergic inhibitory connections in hippocampal transplants. Neuroscience. 1988. 27:373–385.
Article30. Frotscher M, Léránth C, Lübbers K, Oertel WH. Commissural afferents innervate glutamate decarboxylase immunoreactive non-pyramidal neurons in the guinea pig hippocampus. Neurosci Lett. 1984. 46:137–143.
Article31. Grudt TJ, Jahr CE. Quisqualate activates N-methyl-D-aspartate receptor channels in hippocampal neurons maintained in culture. Mol Pharmacol. 1990. 37:477–481.32. Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004. 554:100–110.33. Yang Q, Chen SR, Li DP, Pan HL. Kv1.1/1.2 channels are downstream effectors of nitric oxide on synaptic GABA release to preautonomic neurons in the paraventricular nucleus. Neuroscience. 2007. 149:315–327.
Article34. Soares AC, Duarte ID. Dibutyryl-cyclic GMP induces perip-heral antinociception via activation of ATP-sensitive K(+) channels in the rat PGE2-induced hyperalgesic paw. Br J Pharmacol. 2001. 134:127–131.
Article35. Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999. 83:35C–44C.36. Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999. 83:13C–20C.
Article37. Ishikura F, Beppu S, Hamada T, Khandheria BK, Seward JB, Nehra A. Effects of sildenafil citrate (Viagra) combined with nitrate on the heart. Circulation. 2000. 102:2516–2521.
Article38. Ravipati G, McClung JA, Aronow WS, Peterson SJ, Frishman WH. Type 5 phosphodiesterase inhibitors in the treatment of erectile dysfunction and cardiovascular disease. Cardiol Rev. 2007. 15:76–86.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Opioid Receptor on the Analgesic Action of Intrathecal Sildenafil in Rats
- Role of PKG-L-type calcium channels in the antinociceptive effect of intrathecal sildenafil
- Evaluation for the Effects of Intrathecal Sildenafil on the Formalin- and Thermal-induced Nociception of Rats
- Animal Models for Orofacial Neuropathic Pain
- Additive Antinociception between Intrathecal Sildenafil and Morphine in the Rat Formalin Test