J Korean Med Sci.
2013 Dec;28(12):1716-1722. 10.3346/jkms.2013.28.12.1716.
A Clinical Trial and Extension Study of Infliximab in Korean Patients with Active Rheumatoid Arthritis despite Methotrexate Treatment
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. ysong@snu.ac.kr
- 2Department of Internal Medicine, Gachon University School of Medicine, Incheon, Korea.
- 3Department of Internal Medicine, The Hospital for Rheumatic Diseases, Hanyang University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea.
- KMID: 1779410
- DOI: http://doi.org/10.3346/jkms.2013.28.12.1716
Abstract
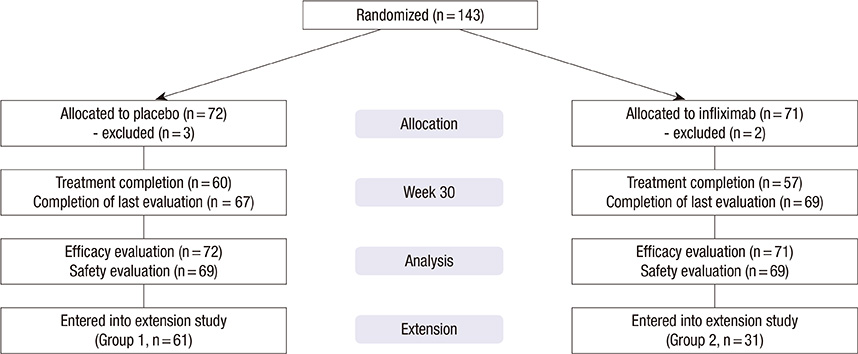
- Currently, infliximab is given for disease control for active rheumatoid arthritis (RA) patients despite methotrexate treatment. However, the efficacy and safety of infliximab in Korean patients has not been assessed appropriately. Therefore, we performed placebo-controlled, double-blind, randomized study and extension study. One-hundred forty-three patients with active RA were randomized to receive placebo or infliximab 3 mg/kg intravenously at week 0, 2, 6, 14, and 22 with methotrexate maintenance. Primary endpoint was American College of Rheumatology 20% improvement criteria (ACR20) at 30 week. After the clinical trial, patients on placebo (Group 1) and patients on infliximab who showed ACR20 response (Group 2) were treated with infliximab through another 84 week for evaluation of safety. During clinical trial, patients in infliximab group showed higher ACR20 at week 30 than patients in placebo group (50.1% vs 30.6%, P=0.014). A total of 92 patients participated in the extension study. The maintenance rate of infliximab was 62.0% at 84 weeks of extension study. The overall rate of adverse events was not different between Group 1 and Group 2. In Korean patients with active RA despite methotrexate treatment, infliximab in combination with methotrexate is effective and the long-term treatment with infliximab is well tolerated. (ClinicalTrials.gov No. NCT00202852, NCT00732875)
Keyword
MeSH Terms
-
Adult
Aged
Antibodies, Monoclonal/*therapeutic use
Antirheumatic Agents/*therapeutic use
Arthritis, Rheumatoid/*drug therapy
Double-Blind Method
Drug Therapy, Combination
Female
Humans
Male
Methotrexate/*therapeutic use
Middle Aged
Placebo Effect
Republic of Korea
Severity of Illness Index
Time Factors
Treatment Outcome
Antibodies, Monoclonal
Antirheumatic Agents
Methotrexate
Figure
Reference
-
1. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003; 423:356–361.2. Feldmann M, Brennan FM, Chantry D, Haworth C, Turner M, Abney E, Buchan G, Barrett K, Barkley D, Chu A. Cytokine production in the rheumatoid joint: implications for treatment. Ann Rheum Dis. 1990; 49:480–486.3. Cope AP, Aderka D, Doherty M, Engelmann H, Gibbons D, Jones AC, Brennan FM, Maini RN, Wallach D, Feldmann M. Increased levels of soluble tumor necrosis factor receptors in the sera and synovial fluid of patients with rheumatic diseases. Arthritis Rheum. 1992; 35:1160–1169.4. Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993; 30:1443–1453.5. Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994; 344:1105–1110.6. Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial: ATTRACT Study Group. Lancet. 1999; 354:1932–1939.7. Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis: Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000; 343:1594–1602.8. Yamanaka H, Tanaka Y, Sekiguchi N, Inoue E, Saito K, Kameda H, Iikuni N, Nawata M, Amano K, Shinozaki M, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM). Mod Rheumatol. 2007; 17:28–32.9. Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekiguchi N, Sato E, Nawata M, Kameda H, Iwata S, Amano K, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2). Mod Rheumatol. 2008; 18:146–152.10. Gao GH, Li J, Xie HW, Lü Z. Therapeutic effect of infliximab on moderate and severe active rheumatoid arthritis. Nan Fang Yi Ke Da Xue Xue Bao. 2010; 30:724–726.11. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988; 31:315–324.12. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992; 35:498–502.13. Paulus HE, Egger MJ, Ward JR, Williams HJ. Analysis of improvement in individual rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs, based on the findings in patients treated with placebo: the Cooperative Systematic Studies of Rheumatic Diseases Group. Arthritis Rheum. 1990; 33:477–484.14. Uppsala Monitoring Centre. The WHO adverse reaction terminology - WHO-ART. accessed on 3 January 2012. Available at http://www.umc-products.com/graphics/3149.pdf.15. Smolen JS, Emery P. Infliximab: 12 years of experience. Arthritis Res Ther. 2011; 13:S2.16. Voulgari PV, Alamanos Y, Nikas SN, Bougias DV, Temekonidis TI, Drosos AA. Infliximab therapy in established rheumatoid arthritis: an observational study. Am J Med. 2005; 118:515–520.17. Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. British Society for Rheumatology Biologics Register. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006; 54:2368–2376.18. Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova-Scholz M, Gromnica-Ihle E, Antoni C, Herzer P, Kekow J, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005; 52:3403–3412.19. Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, Rahman MU. START Study Group. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006; 54:1075–1086.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hyperpigmentation Probably Induced by Methotrexate in a Patient with Rheumatoid Arthritis
- Combination treatment with ldflunomide and methotrexate in patients with rheumatoid arthritis: the efficacy, safety, and predisposing factors for treatment response
- Description of the Efficacy and Safety of Three New Biologics in the Treatment of Rheumatoid Arthritis
- A Case of Methotrexate: Associated Interstitial Pneumonitis in Rheumatoid Arthritis
- The efficacy and safety of etanercept in patients with active rheumatoid arthritis receiving methotrexate