J Korean Med Sci.
2010 Nov;25(11):1646-1651. 10.3346/jkms.2010.25.11.1646.
The Expression of Corticotropin-Releasing Factor in the Central Nucleus of the Amygdala, Induced by Colorectal Distension, is Attenuated by General Anesthesia
- Affiliations
-
- 1Department of Neuropsychiatry, College of Medicine and Institute of Mental Health, Hanyang University, Seoul, Korea. odh@hanyang.ac.kr
- 2Department of Anatomy and Cell Biology, College of Medicine, Hanyang University, Seoul, Korea.
- KMID: 1779236
- DOI: http://doi.org/10.3346/jkms.2010.25.11.1646
Abstract
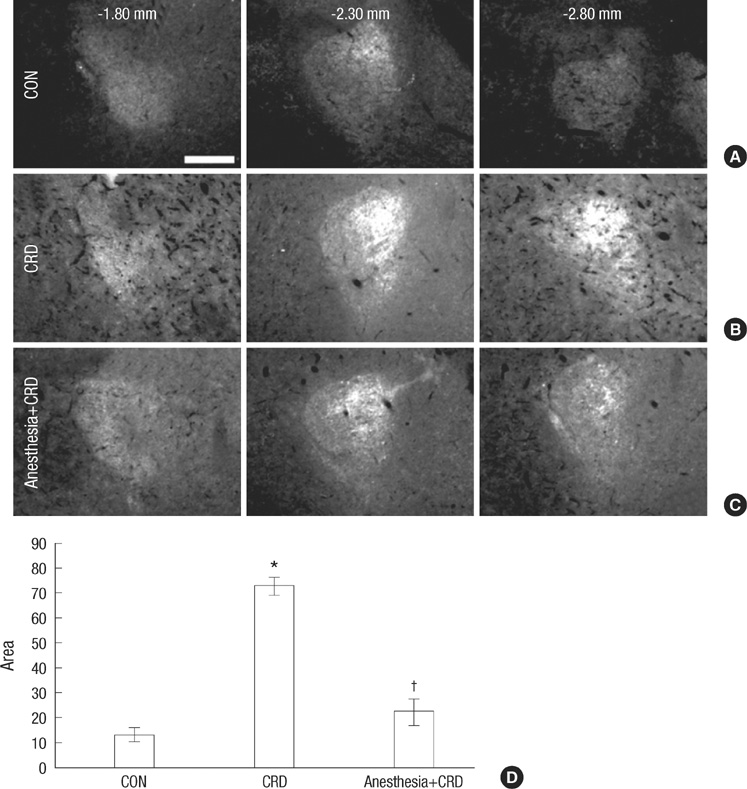
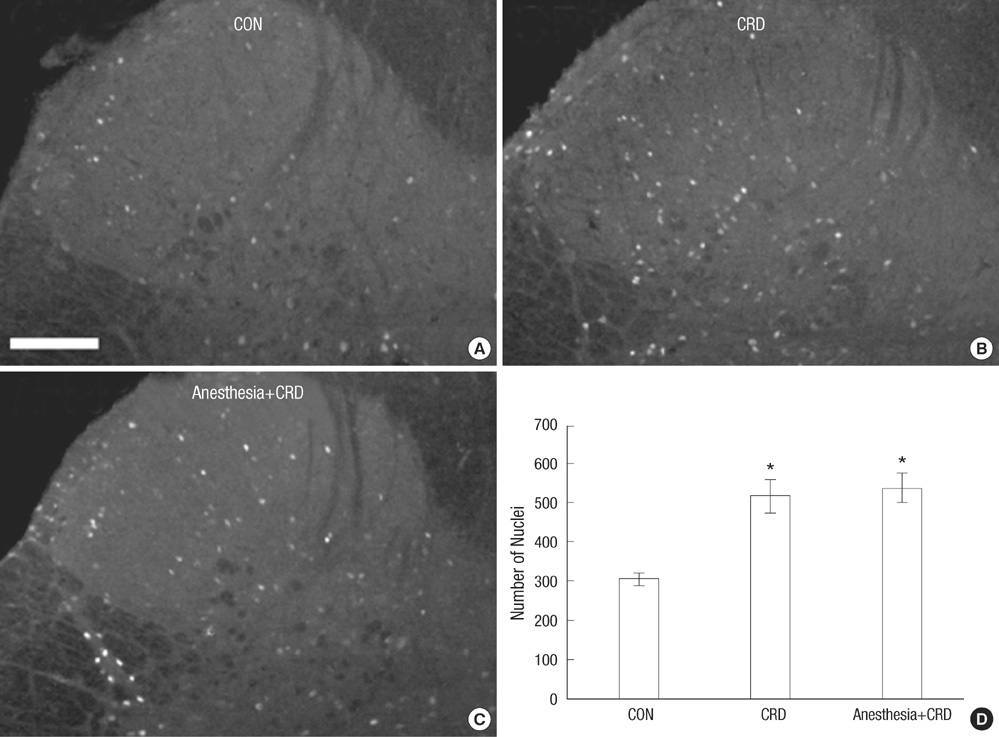
- Corticotrophin-releasing factor (CRF), a key regulator of the hypothalamic-pituitary axis, is expressed in the central nucleus of the amygdala (CeA) and its expression is upregulated in stress-related disorders. We investigated here the effect of noxious colorectal distension (CRD) on the expression of CRF in the CeA of conscious and unconscious rats. Adult male rats with or without general anesthesia were exposed to visceral pain induced by CRD for 5 min; this procedure was repeated 3 times with 1 min resting after each distension. The rats were sacrificed and sections of the CeA were immunostained for CRF as an indicator for anxiety response, and for phosphorylated extracellular signal-regulated kinase (p-ERK) as a marker for pain-specific activation of neurons; sections of lumbosacral spinal cord were immunostained for c-Fos as a marker for activation of spinal neurons. CRD elicited a significant increase in the expression of CRF and p-ERK in the CeA and of c-Fos in the spinal cord. General anesthesia attenuated the increase in CRF and p-ERK in the CeA, but did not affect the expression of spinal c-Fos. These results suggest that conscious recognition of pain at higher brain centers is an important determinant of CRF expression in the CeA.
MeSH Terms
-
Amygdala/*metabolism/pathology
*Anesthesia, General
Animals
Colon
Corticotropin-Releasing Hormone/*metabolism
Extracellular Signal-Regulated MAP Kinases/metabolism
Immunohistochemistry
Male
Neurons/metabolism
Pain/prevention & control
Phosphorylation
Proto-Oncogene Proteins c-fos/metabolism
Rats
Rats, Sprague-Dawley
Rectum
Figure
Reference
-
1. Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol. 2008. 99:1201–1212.
Article2. Nemeroff CB. Psychopharmacology of affective disorders in the 21st century. Biol Psychiatry. 1998. 44:517–525.3. Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984. 226:1342–1344.
Article4. Baker DG, West SA, Nicholson WE, Ekhator NN, Kasckow JW, Hill KK, Bruce AB, Orth DN, Geracioti TD Jr. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1999. 156:585–588.5. Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, Nemeroff CB, Charney DS. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997. 154:624–629.6. Sautter FJ, Bissette G, Wiley J, Manguno-Mire G, Schoenbachler B, Myers L, Johnson JE, Cerbone A, Malaspina D. Corticotropin-releasing factor in posttraumatic stress disorder (PTSD) with secondary psychotic symptoms, nonpsychotic PTSD, and healthy control subjects. Biol Psychiatry. 2003. 54:1382–1388.
Article7. Golianu B, Bhandari R, Shaw JR, Borse GW, Gaeta R, Spiegel D. Yudofsky CS, Hales ER, editors. Neuropsychiatric aspects of pain management. The American psychiatric publishing textbook of neuropsychiatry and behavioral neurosciences. 2008. 5th ed. Arlington, VA: American Psychiatric Publishing;368–369.8. Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992. 15:353–375.
Article9. Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004. 10:221–234.
Article10. Traub RJ, Silva E, Gebhart GF, Solodkin A. Noxious colorectal distention induced-c-Fos protein in limbic brain structures in the rat. Neurosci Lett. 1996. 215:165–168.
Article11. Wang L, Martinez V, Larauche M, Tache Y. Proximal colon distension induces Fos expression in oxytocin-, vasopressin-, CRF- and catecholamines-containing neurons in rat brain. Brain Res. 2009. 1247:79–91.
Article12. Zhang XJ, Li Z, Chung EK, Zhang HQ, Xu HX, Sung JJ, Bian ZX. Activation of extracellular signal-regulated protein kinase is associated with colorectal distension-induced spinal and supraspinal neuronal response and neonatal maternal separation-induced visceral hyperalgesia in rats. J Mol Neurosci. 2009. 37:274–287.
Article13. Martinez V, Wang L, Tache Y. Proximal colon distension induces Fos expression in the brain and inhibits gastric emptying through capsaicin-sensitive pathways in conscious rats. Brain Res. 2006. 1086:168–180.14. Saito-Nakaya K, Hasegawa R, Nagura Y, Ito H, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks colonic hypersensitivity induced by a combination of inflammation and repetitive colorectal distension. Neurogastroenterol Motil. 2008. 20:1147–1156.
Article15. Trimble N, Johnson AC, Foster A, Greenwood-van Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastroenterol Motil. 2007. 19:754–760.
Article16. Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor 1 antagonist blocks brain-gut activation induced by colonic distention in rats. Gastroenterology. 2005. 129:1533–1543.
Article17. Greenwood-Van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil. 2005. 17:415–422.
Article18. Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn Reson Med. 1999. 41:412–416.
Article19. Lazovic J, Wrzos HF, Yang QX, Collins CM, Smith MB, Norgren R, Matyas K, Ouyang A. Regional activation in the rat brain during visceral stimulation detected by c-fos expression and fMRI. Neurogastroenterol Motil. 2005. 17:548–556.
Article20. Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004. 1000:40–56.
Article21. Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988. 450:153–169.
Article22. Eitner F, Bucher E, van Roeyen C, Kunter U, Rong S, Seikrit C, Villa L, Boor P, Fredriksson L, Backstrom G, Eriksson U, Ostman A, Floege J, Ostendorf T. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J Am Soc Nephrol. 2008. 19:281–289.
Article23. Wang Z, Bradesi S, Maarek JM, Lee K, Winchester WJ, Mayer EA, Holschneider DP. Regional brain activation in conscious, nonrestrained rats in response to noxious visceral stimulation. Pain. 2008. 138:233–243.
Article24. Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997. 20:517–523.25. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000. 23:155–184.
Article26. Cohen-Matsliah SI, Brosh I, Rosenblum K, Barkai E. A novel role for extracellular signal-regulated kinase in maintaining long-term memory-relevant excitability changes. J Neurosci. 2007. 27:12584–12589.
Article27. Di Benedetto B, Kallnik M, Weisenhorn DM, Falls WA, Wurst W, Holter SM. Activation of ERK/MAPK in the lateral amygdala of the mouse is required for acquisition of a fear-potentiated startle response. Neuropsychopharmacology. 2009. 34:356–366.
Article28. Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000. 20:8177–8187.
Article29. Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995. 15:5439–5447.30. Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, Wilson MA. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res. 2002. 949:122–130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Repeated Stress on Expression of Corticotropin Releasing Factor Type I and II Receptors
- Neurobiology of Depression
- Immunohistochemical study on the somatostatin and corticotropin-releasing factor neurons in the hypothalamus of the dog
- The Prediction of Preterm Labor : The Role of Corticotropin-Releasing Hormone in Amniotic Fluid
- Enhancement of Nitric Oxide Production by Corticotropin-releasing Hormone (CRH) in Murine Microglial Cells, BV2