J Korean Med Sci.
2010 Nov;25(11):1626-1632. 10.3346/jkms.2010.25.11.1626.
Exendin-4 Protects Oxidative Stress-Induced beta-Cell Apoptosis through Reduced JNK and GSK3beta Activity
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Konyang University School of Medicine, Daejeon, Korea. kbjoon4u@hananet.net
- 2Department of Cardiology, Gachon University of Medicine and Science, Incheon, Korea.
- 3Department of Biochemistry and Molecular Biology, Eulji University School of Medicine, Daejeon, Korea.
- KMID: 1779233
- DOI: http://doi.org/10.3346/jkms.2010.25.11.1626
Abstract
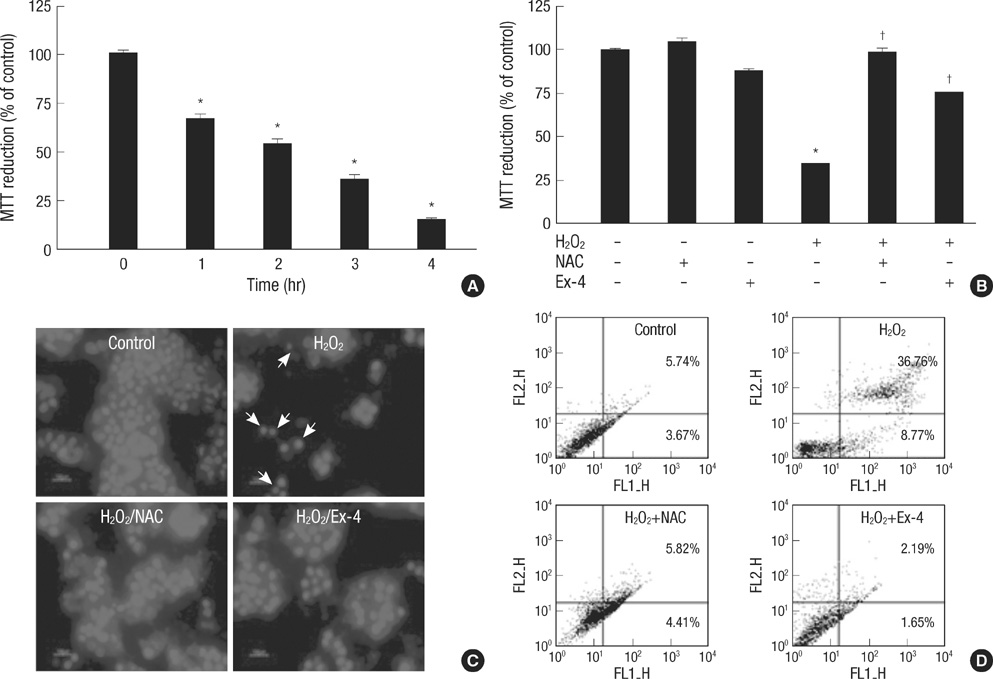
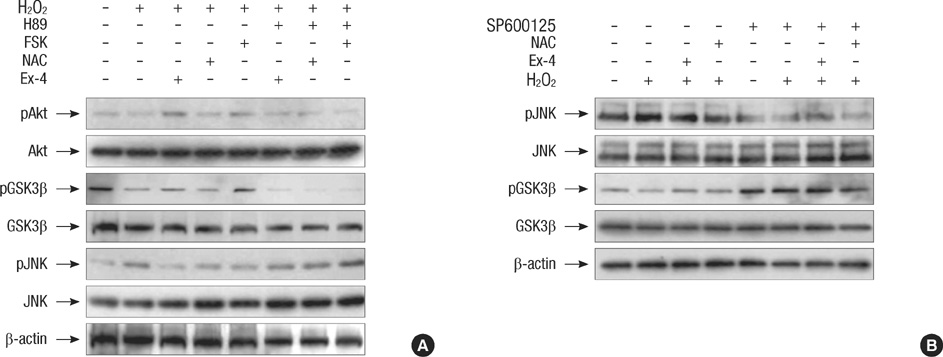
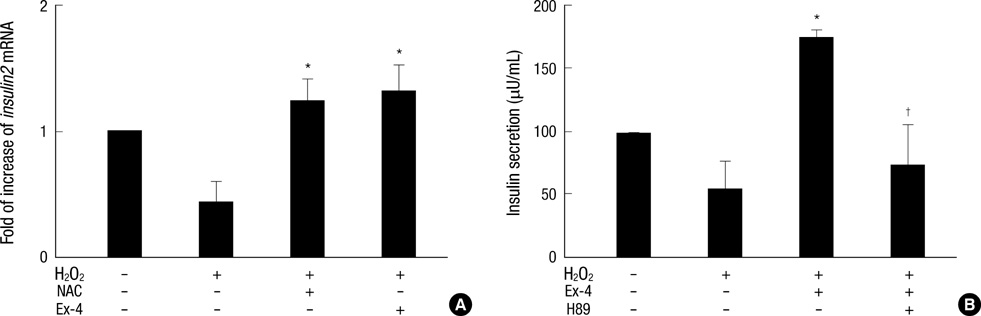
- Oxidative stress induced by chronic hyperglycemia in type 2 diabetes plays a crucial role in progressive loss of beta-cell mass through beta-cell apoptosis. Glucagon like peptide-1 (GLP-1) has effects on preservation of beta-cell mass and its insulin secretory function. GLP-1 possibly increases islet cell mass through stimulated proliferation from beta-cell and differentiation to beta-cell from progenitor cells. Also, it probably has an antiapoptotic effect on beta-cell, but detailed mechanisms are not proven. Therefore, we examined the protective mechanism of GLP-1 in beta-cell after induction of oxidative stress. The cell apoptosis decreased to ~50% when cells were treated with 100 microM H2O2 for up to 2 hr. After pretreatment of Ex-4, GLP-1 receptor agonist, flow cytometric analysis shows 41.7% reduction of beta-cell apoptosis. This data suggested that pretreatment of Ex-4 protect from oxidative stress-induced apoptosis. Also, Ex-4 treatment decreased GSK3beta activation, JNK phosphorylation and caspase-9, -3 activation and recovered the expression of insulin2 mRNA in beta-cell lines and secretion of insulin in human islet. These results suggest that Ex-4 may protect beta-cell apoptosis by blocking the JNK and GSK3beta mediated apoptotic pathway.
MeSH Terms
-
Animals
*Apoptosis
Caspase 3/metabolism
Caspase 9/metabolism
Cells, Cultured
Cricetinae
Flow Cytometry
Glucagon-Like Peptide 1/pharmacology
Glycogen Synthase Kinase 3/*metabolism
Humans
Hydrogen Peroxide/toxicity
Insulin/genetics/metabolism
Insulin-Secreting Cells/drug effects/*enzymology/metabolism
JNK Mitogen-Activated Protein Kinases/*metabolism
*Oxidative Stress
Peptides/*pharmacology
Phosphorylation
Receptors, Glucagon/agonists/metabolism
Signal Transduction
Venoms/*pharmacology
Figure
Reference
-
1. Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patanè G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999. 274:14112–14121.2. Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008. 9:329–343.3. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000. 23:1084–1091.4. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.
Article5. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002. 23:599–622.
Article6. Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death. Science. 2005. 307:380–384.7. King GL, Brownlee M. The cellular and molecular mechanisms of diabetic complications. Endocrinol Metab Clin North Am. 1996. 25:255–270.
Article8. Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001. 17:189–212.9. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Matsuhisa M, Yamasaki Y. Oxidative stress and the JNK pathway in diabetes. Curr Diabetes Rev. 2005. 1:65–72.
Article10. Kaneto H, Matsuoka TA, Nakatani Y, Kawamori D, Miyatsuka T, Matsuhisa M, Yamasaki Y. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J Mol Med. 2005. 83:429–439.
Article11. Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002. 277:30010–30018.
Article12. Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001. 7:1133–1137.13. Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001. 108:1631–1638.14. Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995. 378:785–789.
Article15. Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007. 282:12030–12037.
Article16. Liu Z, Tanabe K, Bernal-Mizrachi E, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008. 51:623–631.17. Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007. 113:546–593.
Article18. Park IB. New and emerging drugs in type 2 diabetes. Korean J Med. 2007. 72:446–450.19. Kim BJ. Stimulation of glucagon like peptide-1 secretion in enteroendocrine L cells. Korean Diabetes J. 2009. 33:458–463.
Article20. Kim JY, Lee SK, Baik HW, Lee KH, Kim HJ, Park KS, Kim BJ. Protective effects of glucagon like peptide-1 on HIT-T15 beta cell apoptosis via ER stress induced by 2-deoxy-D-glucose. Korean Diabetes J. 2008. 32:477–487.21. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000. 343:230–238.
Article22. Steensma DP, Timm M, Witzig TE. Flow cytometric methods for detection and quantification of apoptosis. Methods Mol Med. 2003. 85:323–332.23. Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002. 7:313–319.24. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008. 118:3378–3389.25. Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001. 359:1–16.
Article26. Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000. 275:10761–10766.
Article27. Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 2001. 78:1219–1232.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Padina arborescens extract protects high glucose-induced apoptosis in pancreatic beta cells by reducing oxidative stress
- Beta Cells Preservation in Diabetes using GLP-1 and Its Analog
- Resveratrol Protects HepG2 and Chang Liver Cells from Oxidative Stress
- Purpurogallin Protects Keratinocytes from Damage and Apoptosis Induced by Ultraviolet B Radiation and Particulate Matter 2.5
- The mechanism of t-butylhydroperoxide-induced apoptosis in IMR-32 human neuroblastoma cells