J Korean Med Sci.
2009 Aug;24(4):729-736. 10.3346/jkms.2009.24.4.729.
Comparative Study of the Effects of Different Growth Hormone Doses on Growth and Spatial Performance of Hypophysectomized Rats
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. jindk@skku.edu
- 2Department of Pediatrics, College of Medicine, Pusan National University, Busan, Korea.
- 3Laboratory Animal Research Center, Samsung Biomedical Research Institute, Seoul, Korea.
- KMID: 1779211
- DOI: http://doi.org/10.3346/jkms.2009.24.4.729
Abstract
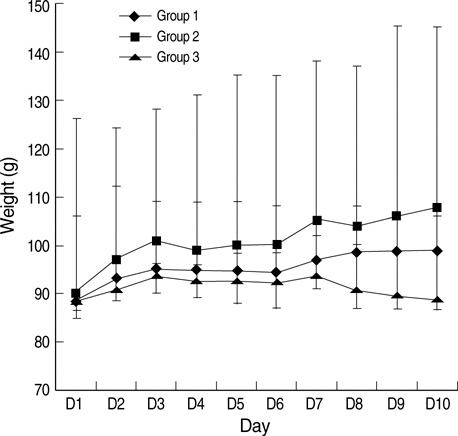
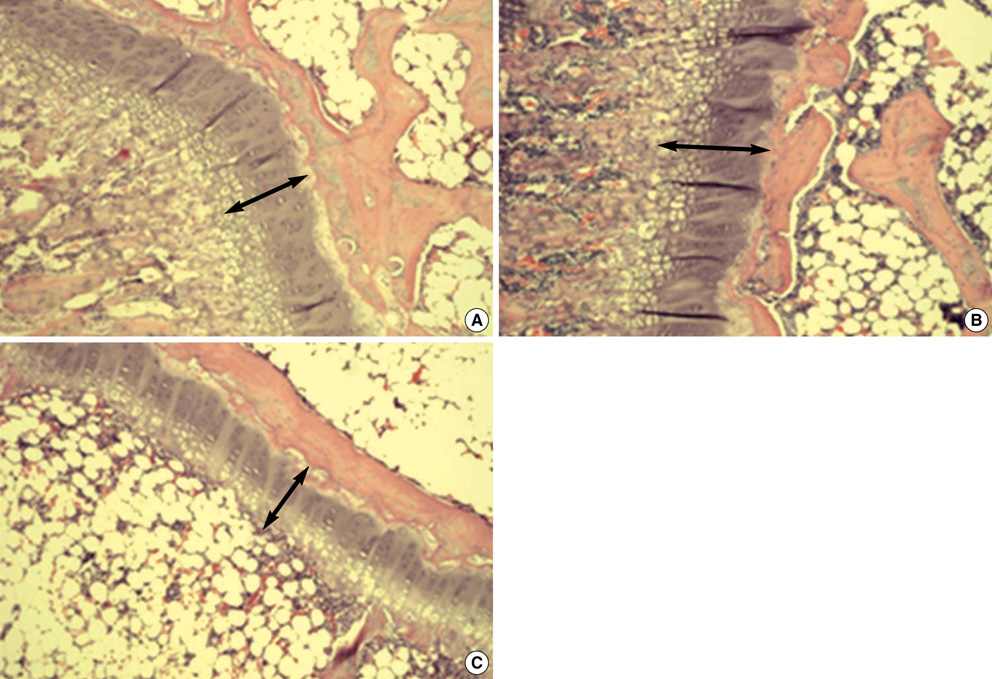
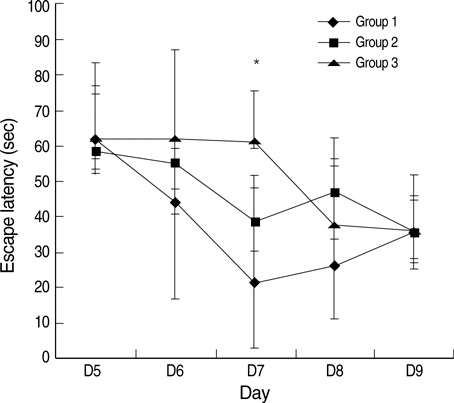
- This study was designed to examine the effects of recombinant human growth hormone replacement on somatic growth and cognitive function in hypophysectomized (HYPOX) female Sprague-Dawley rats. Rats (5 per group) were randomized by weight to 3 experimental groups: group 1, administered 200 microgram/kg of GH once daily for 9 days; group 2, administered 200 microgram/kg of GH twice daily; and group 3, administered saline daily. Somatic growth was evaluated by measurement of body weight daily and of the width of the proximal tibial growth plate of the HYPOX rats. Cognitive function was evaluated using the Morris water maze (MWM) test. The results indicated that GH replacement therapy in HYPOX rats promoted an increase in the body weight and the width of the tibial growth plate in a dose-dependent manner. On the third day of the MWM test, the escape latency in the GH-treated groups 1 and 2 was significantly shorter than that in the control rats (P<0.001 and P=0.032, respectively), suggesting that rhGH improved spatial memory acquisition in the MWM test. Therefore it is concluded that rhGH replacement therapy in HYPOX rats stimulates an increase in somatic growth in a dose-dependent manner and also has beneficial effects on cognitive functions.
Keyword
MeSH Terms
Figure
Reference
-
1. Hindmarsh PC, Dattani MT. Use of growth hormone in children. Nat Clin Pract Endocrinol Metab. 2006. 2:260–268.
Article2. Underwood LE, Attie KM, Baptista J. Growth hormone (GH) dose-response in young adults with childhood-onset GH deficiency: a two-year, multicenter, multiple-dose, placebo-controlled study. J Clin Endocrinol Metab. 2003. 88:5273–5280.
Article3. Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tolli J, Sjostrom L, Isaksson OG. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993. 76:309–317.
Article4. O'Halloran DJ, Tsatsoulis A, Whitehouse RW, Holmes SJ, Adams JE, Shalet SM. Increased bone density after recombinant human growth hormone (GH) therapy in adults with isolated GH deficiency. J Clin Endocrinol Metab. 1993. 76:1344–1348.5. Cuneo RC, Salomon F, Watts GF, Hesp R, Sonksen PH. Growth hormone treatment improves serum lipids and lipoproteins in adults with growth hormone deficiency. Metabolism. 1993. 42:1519–1523.
Article6. Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989. 321:1797–1803.
Article7. Gibney J, Wallace JD, Spinks T, Schnorr L, Ranicar A, Cuneo RC, Lockhart S, Burnand KG, Salomon F, Sonksen PH, Russell-Jones D. The effects of 10 years of recombinant human growth hormone (GH) in adult GH-deficient patients. J Clin Endocrinol Metab. 1999. 84:2596–2602.
Article8. Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998. 23:45–55.
Article9. Sathiavageeswaran M, Burman P, Lawrence D, Harris AG, Falleti MG, Maruff P, Wass J. Effects of GH on cognitive function in elderly patients with adult-onset GH deficiency: a placebo-controlled 12-month study. Eur J Endocrinol. 2007. 156:439–447.
Article10. van Dam PS, Aleman A, de Vries WR, Deijen JB, van der Veen EA, de Haan EH, Koppeschaar HP. Growth hormone, insulin-like growth factor I and cognitive function in adults. Growth Horm IGF Res. 2000. 10:Suppl B. S69–S73.11. Aleman A, Verhaar HJ, De Haan EH, De Vries WR, Samson MM, Drent ML, Van der Veen EA, Koppeschaar HP. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999. 84:471–475.
Article12. Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006. 27:318–323.
Article13. Schneider-Rivas S, Rivas-Arancibia S, Vazquez-Pereyra F, Vazquez-Sandoval R, Borgonio-Perez G. Modulation of long-term memory and extinction responses induced by growth hormone (GH) and growth hormone releasing hormone (GHRH) in rats. Life Sci. 1995. 56:PL433–PL441.
Article14. Le Greves M, Zhou Q, Berg M, Le Greves P, Fholenhag K, Meyerson B, Nyberg F. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp Brain Res. 2006. 173:267–273.
Article15. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neuropsychiatry Clin Neurosci. 2000. 12:103–113.
Article16. Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982. 297:681–683.
Article17. Cox GN, Rosendahl MS, Chlipala EA, Smith DJ, Carlson SJ, Doherty DH. A long-acting, mono-PEGylated human growth hormone analog is a potent stimulator of weight gain and bone growth in hypophysectomized rats. Endocrinology. 2007. 148:1590–1597.
Article18. Nagamine J, Nakagawa T, Taiji M. Recombinant human growth hormone (SMP-140) is effective for growth promotion in hypophysectomized rats. Biomed Res. 2006. 27:191–195.
Article19. Gargosky SE, Tapanainen P, Rosenfeld RG. Administration of growth hormone (GH), but not insulin-like growth factor-I (IGF-I), by continuous infusion can induce the formation of the 150-kilodalton IGF-binding protein-3 complex in GH-deficient rats. Endocrinology. 1994. 134:2267–2276.
Article20. Bick T, Amit T, Barkey RJ, Hertz P, Youdim MB, Hochberg Z. The interrelationship of growth hormone (GH), liver membrane GH receptor, serum GH-binding protein activity, and insulin-like growth factor I in the male rat. Endocrinology. 1990. 126:1914–1920.
Article21. Chapman IM, Helfgott A, Willoughby JO. Disappearance half-life times of exogenous and growth hormone-releasing factor-stimulated endogenous growth hormone in normal rats. J Endocrinol. 1991. 128:369–374.
Article22. MacGillivray MH, Baptista J, Johanson A. Outcome of a four-year randomized study of daily versus three times weekly somatropin treatment in prepubertal naive growth hormone-deficient children. Genentech Study Group. J Clin Endocrinol Metab. 1996. 81:1806–1809.
Article23. Gevers EF, van der Eerden BC, Karperien M, Raap AK, Robinson IC, Wit JM. Localization and regulation of the growth hormone receptor and growth hormone-binding protein in the rat growth plate. J Bone Miner Res. 2002. 17:1408–1419.
Article24. Werther GA, Haynes K, Edmonson S, Oakes S, Buchanan CJ, Herington AC, Waters MJ. Identification of growth hormone receptors on human growth plate chondrocytes. Acta Paediatr Suppl. 1993. 82:suppl 391. 50–53.
Article25. Hoffman DM, Crampton L, Sernia C, Nguyen TV, Ho KK. Short-term growth hormone (GH) treatment of GH-deficient adults increases body sodium and extracellular water, but not blood pressure. J Clin Endocrinol Metab. 1996. 81:1123–1128.
Article26. Stevens DA, Williams GR. Hormone regulation of chondrocyte differentiation and endochondral bone formation. Mol Cell Endocrinol. 1999. 151:195–204.
Article27. Isaksson OG, Lindahl A, Nilsson A, Isgaard J. Mechanism of the stimulatory effect of growth hormone on longitudinal bone growth. Endocr Rev. 1987. 8:426–438.
Article28. Wang J, Zhou J, Bondy CA. Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 1999. 13:1985–1990.
Article29. Woods KA, Camacho-Hubner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996. 335:1363–1367.
Article30. van der Reijden-Lakeman IE, de Sonneville LM, Swaab-Barneveld HJ, Slijper FM, Verhulst FC. Evaluation of attention before and after 2 years of growth hormone treatment in intrauterine growth retarded children. J Clin Exp Neuropsychol. 1997. 19:101–118.31. Rollero A, Murialdo G, Fonzi S, Garrone S, Gianelli MV, Gazzerro E, Barreca A, Polleri A. Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology. 1998. 38:73–79.
Article32. Lai Z, Roos P, Zhai O, Olsson Y, Fholenhag K, Larsson C, Nyberg F. Age-related reduction of human growth hormone-binding sites in the human brain. Brain Res. 1993. 621:260–266.
Article33. Johansson JO, Larson G, Andersson M, Elmgren A, Hynsjo L, Lindahl A, Lundberg PA, Isaksson OG, Lindstedt S, Bengtsson BA. Treatment of growth hormone-deficient adults with recombinant human growth hormone increases the concentration of growth hormone in the cerebrospinal fluid and affects neurotransmitters. Neuroendocrinology. 1995. 61:57–66.
Article34. LeRoith D, Werner H, Beitner-Johnson D, Roberts CT Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995. 16:143–163.
Article35. Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998. 87:559–569.
Article36. Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996. 87:1327–1338.
Article37. Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMD-Aantagonist D-AP5. Hippocampus. 1999. 9:118–136.
Article38. Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999. 401:63–69.
Article39. Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001. 432:230–243.
Article40. Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007. 7:39–47.
Article41. Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signalling. Trends Cell Biol. 2000. 10:274–280.
Article42. Le Greves M, Steensland P, Le Greves P, Nyberg F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-D-aspartate receptor subunits gene transcripts in male rats. Proc Natl Acad Sci U S A. 2002. 99:7119–7123.
Article43. Le Greves M, Le Greves P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005. 65:369–374.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Growth Hormone Tretment on Body Composition and Glucose Metabolism in Adult Hypophysectomized Rats
- Effect of Human Growth Hormone to Antioxidant Enzymes in Rat Erythrocyte
- Clinical effects of yeast derived recombinant methionyl growth hormone in growth hormone deficiency
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- The Growth Hormone Response to Growth Hormone-Releasing Hexapeptide (GHRP-6) in the Sprague Dawley Rat with Hypothyroidism