J Korean Med Sci.
2004 Dec;19(6):820-825. 10.3346/jkms.2004.19.6.820.
Phase II Study of Cyclophosphamide, Epirubicin, Vincristine, Prednisone, and Etoposide (CEOP-E) for Aggressive Non-Hodgkin 's Lymphoma
- Affiliations
-
- 1Department of Hematology/Oncology, Kyungpook National University Hospital, Daegu, Korea. sksohn@knu.ac.kr
- 2Department of Pathology, Kyungpook National University Hospital, Daegu, Korea.
- KMID: 1778563
- DOI: http://doi.org/10.3346/jkms.2004.19.6.820
Abstract
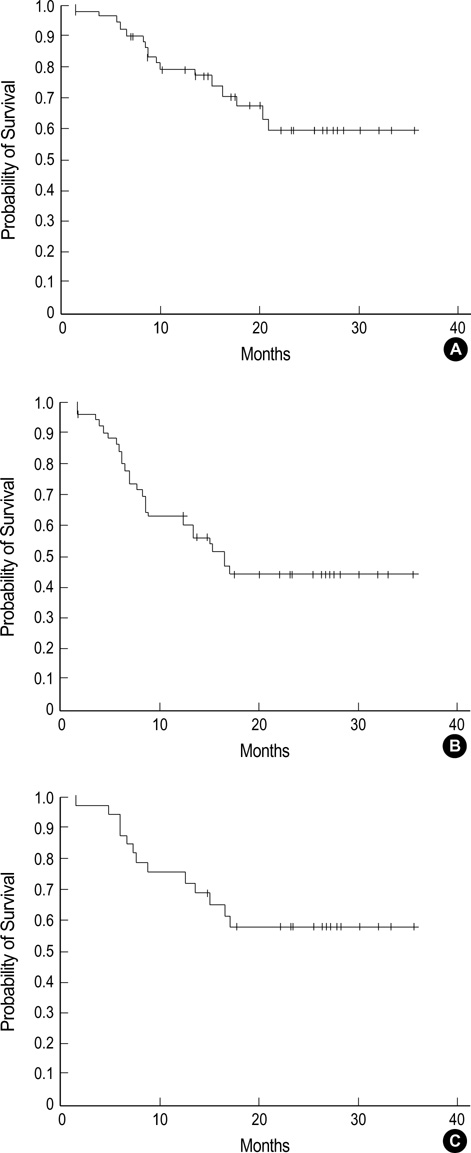
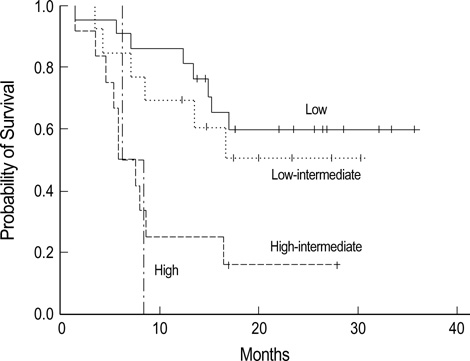
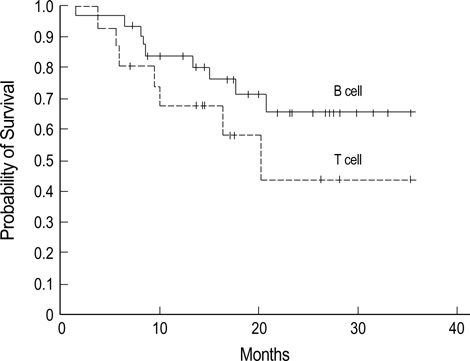
- The main objectives of the current study were to evaluate the efficacy and safety of a CEOP-E regimen for patients with aggressive non-Hodgkin's lymphoma (NHL). Fifty-one consecutive patients with newly diagnosed aggressive NHL were enrolled in the study. Median age of patients was 57 (range, 18-75) yr old, and male to female ratio was 1.32:1. Diffuse large B cell lymphoma (68.8%) was the most common histological subtype. Thirty patients (58.8%) had Ann Arbor stage III or IV diseases at diagnosis. One course of chemotherapy consisted of an intravenous combination of cyclophosphamide 750 mg/m2, epirubicin 50 mg/m2, vincristine 2 mg, etoposide 80 mg/m 2 on day 1 and oral administration of 100 mg prednisone on days 1 to 5 (CEOP-E). A complete response or unconfirmed complete response was achieved in 31 (63.3%) out of 49 evaluated patients. With a median follow-up of 16.3 months, 26 events including relapse and death were observed. The estimated 2-yr survival rate for all patients and disease free survival rate for patients achieving complete re-sponse was 58.9% and 57.1%, respectively. Episodes of febrile neutropenia occurred in 5 (10.2%) patients. Transient episodes of ECG abnormality (1st degree AV block) were observed in 2 patients. Accordingly, the CEOP-E regimen produced comparable results to those of other regimens, including CHOP, in terms of the response rate and overall survival. The current regimen seemed to minimize the cardiac toxicity due to an accumulated dose of anthracycline in the treatment of aggressive NHL.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Antineoplastic Agents/administration & dosage
Antineoplastic Combined Chemotherapy Protocols/*administration & dosage
Cyclophosphamide/*administration & dosage
Epirubicin/*administration & dosage
Etoposide/*administration & dosage
Female
Humans
Lymphoma, Non-Hodgkin/*drug therapy/*mortality
Male
Middle Aged
Prednisone/*administration & dosage
Risk Assessment/*methods
Risk Factors
Survival Analysis
Treatment Outcome
Vincristine/*administration & dosage
Figure
Reference
-
1. Gordon LI, Harrington D, Andersen J, Colgan J, Glick J, Neiman R, Mann R, Resnick GD, Barcos M, Gottlieb A. Comparison of a second generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin's lymphoma. N Engl J Med. 1992. 327:1342–1349.2. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, Glick JH, Coltman CA Jr, Miller TP. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993. 328:1002–1006.
Article3. Longo DL, DeVita VT Jr, Duffey PL, Wesley MN, Ihde DC, Hubbard SM, Gilliom M, Jaffe ES, Cossman J, Fisher RI. Superiority of ProMACE-CytaBOM over ProMACE-MOPP in the treatment of advanced diffuse aggressive lymphoma: results of a prospective randomized trial. J Clin Oncol. 1991. 9:25–38.
Article4. Shipp MA, Yeap BY, Harrington DP, Klatt MM, Pinkus GS, Jochelson MS, Rosenthal OS, Skarin AT, Canellos GP. The m-BACOD combination chemotherapy regimen in large-cell lymphoma: analysis of the completed trial and comparison with M-BACOD regimen. J Clin Oncol. 1990. 8:84–93.5. Koppler H, Pfluger KH, Eschenbach I, Pfab R, Lennert K, Wellens W, Schmidt M, Gassel WD, Kolb T, Hassler R. CHOP-VP16 chemotherapy and involved field irradiation for high grade non-Hodgkin's lymphomas: a phase II multicentre study. Br J Cancer. 1989. 60:79–82.
Article6. Karakas T, Bergmann L, Stutte HJ, Jager E, Knuth A, Weidmann E, Mitrou PS, Hoelzer D. Peripheral T-cell lymphomas respond well to vincristine, adriamycin, cyclophosphamide, prednisone and etoposide (VACPE) and have a similar outcome as high-grade B-cell lymphomas. Leuk lymphoma. 1996. 24:121–129.
Article7. Lambertenghi Deliliers G, Butti C, Baldini L, Ceriani A, Lombardi F, Luoni M, Montalbetti L, Pavia G, Pinotti G, Pogliani E, Cassi E, Pisoni GB. A cooperative study of epirubicin with cyclophosphamide, vincristine and prednisone (CEOP) in non-Hodgkin's lymphoma. Haematologica. 1995. 80:318–324.8. De Lena M, Maiello E, Lorusso V, Brandi M, Calabrese P, Romito S, Mazzei A, Marzullo F. Comparison of CHOP-B vs CEOP-B in poor prognosis non-Hodgkin's lymphomas. A randomized trial. Med Oncol Tumor Pharmacother. 1989. 6:163–169.
Article9. Chim CS, Kwong YL, Lie AK, Lee CK, Liang R. CEOP treatment results and validity of the international prognostic index in Chinese patients with aggressive non-Hodgkin's lymphoma. Hematol Oncol. 1998. 16:117–123.
Article10. Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B, Gatter KC. A revised European-American classification of lymphoid neoplasms: a proposal from the international lymphoma study group. Blood. 1994. 84:1361–1392.11. The International Non-Hodgkin's Lymphoma Prognostic Factor Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993. 329:987–994.12. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-Lopez A, Hagenbeek A, Cabanillas F, Klippensten D, Hiddemann W, Castellino R, Harris NL, Armitage JO, Carter W, Hoppe R, Canellos GP. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999. 17:1244.
Article13. Armitage JO, Fyfe MA, Lewis J. Long-term remission durability and functional status of patients treated for diffuse histiocytic lymphoma with the CHOP regimen. J Clin Oncol. 1984. 2:898–902.
Article14. Gams RA, Rainey M, Dandy M, Bartolucci AA, Silberman H, Omura G. Phase III study of BCOP versus CHOP in unfavorable categories of malignant lymphoma: a Southeastern Cancer Study Group trial. J Clin Oncol. 1985. 3:1188–1195.15. Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998. 339:21–26.
Article16. Melnyk A, Rodriguez A, Pugh WC, Cabannillas F. Evaluation of the Revised European-American Lymphoma Classification confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin's lymphoma. Blood. 1997. 89:4514–4520.
Article17. Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Briere J, Haioun C, Cazals-Hatem D, Bosly A, Xerri L, Tilly H, Berger F, Bouhabdallah R, Diebold J. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Blood. 1998. 92:76–82.18. Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rudolph C, Reiser M, Hossfeld DK, Metzner B, Hasenclever D, Schmitz N, Glass B, Rube C, Loeffler M. 2-weekly vs. 3-weekly CHOP with and without etoposide in young patients with low-risk (low LDH) aggressive non-Hodgkin's lymphoma: Results of the Completed NHL-B-1 Trial of the DSHNHL. Blood. 2002. 100:92a. [abstract].19. Haddy TB, Adde MA, McCalla J, Domanski MJ, Datiles M 3rd, Meehan SC, Pikus A, Shad AT, Valdez I, Lopez Vivino L, Magrath IT. Late effects in long term survivors of high grade non-Hodgkin's lymphomas. J Clin Oncol. 1998. 16:2070–2079.20. Basaran M, Bavbek ES, Sakar B, Eralp Y, Alici S, Tas F, Yaman F, Dogan O, Camlica H, Onat H. Treatment of aggressive non-Hodgkin's lymphoma with dose-intensified epirubicin in combination of cyclophosphamide, vincristine, and prednisone (CEOP-100); A phase II study. Am J Clin Oncol. 2001. 24:570–575.21. Mugitani A, Tatsumi Y, Tanaka K, Yasui Y, Inoue T. Cyclophosphamide, epirubicin, vincristine, prednisone, bleomycin, etoposide (CEOP-BE) therapy for intermediate- and high grade non-Hodgkin's lymphomas. Anticancer Res. 1999. 19:3393–3397.22. Nair R, Ramakrishnan G, Nair NN, Saikia TK, Parikh PM, Joshi SR, Soman CS, Mukhadan M, Dinshaw KT, Advani SH. A randomized comparison of the efficacy and toxicity of epirubicin and doxorubicin in the treatment of patients with non-Hodgkin's lymphoma. Cancer. 1998. 82:2282–2288.
Article23. Case DC, Gams R, Ervin TJ, Boyd MA, Oldham FB. Phase I-II trial of high-dose epirubicin in patients with lymphoma. Cancer Res. 1987. 47:6393–6396.24. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002. 346:235–242.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Advanced Hodgkin Lymphoma
- A Case of Non-Hodgkin Lymphoma during Pregnancy
- Results of CHOP-Bleo / CMED Alternating Chemotherapy for Aggressive Non - Hodgkin's Lymphoma
- A case of primary lymphoma of the prostate presented with symptoms of lower urinary tract obstruction
- Primary diffuse large B-cell lymphoma of the bone marrow in a frail and elderly patient successfully treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone