J Korean Med Sci.
2004 Dec;19(6):793-799. 10.3346/jkms.2004.19.6.793.
Common Whelk (Buccinum undatum)Allergy:Identification of IgE-binding Components and Effects of Heating and Digestive Enzymes
- Affiliations
-
- 1Department of Internal Medicine, Sungkyunkwan University College of Medicine, Suwon, Korea.
- 2Department of Allergy-Rheumatology, Ajou University School of Medicine, Suwon, Korea. hspark@madang.ajou.ac.kr
- KMID: 1778558
- DOI: http://doi.org/10.3346/jkms.2004.19.6.793
Abstract
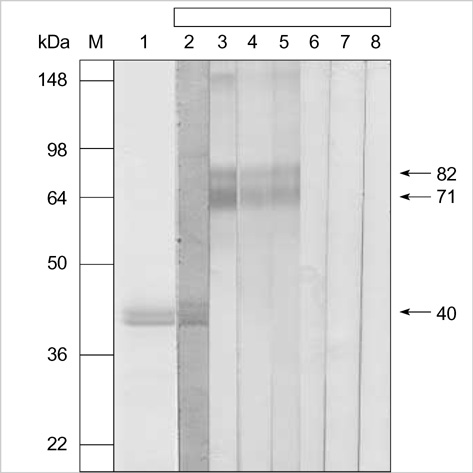
- In Korea, common whelk (Buccinum undatum) is a popular edible shellfish. The aim of this study was to observe the sensitization rate to common whelk and to characterize its allergens. We carried out skin prick test (SPT) in 1,700 patients with various allergic diseases. Specific IgE were detected by ELISA in the patient sera and ELISA inhibition tests were conducted. IgE-binding components were identified by means of SDS-PAGE and IgE-immunoblotting. The effects of diges-tive enzymes were evaluated in both raw and thermally treated extracts. SPT to common whelk was positive (> or =2+) in 83 (4.9%) patients studied. Twenty-four (38.7%) out of 62 SPT positive patients had high serum specific IgE to common whelk. ELISA inhibition test showed significant inhibitions by abalone as well as by common whelk. IgE-immunoblotting demonstrated three IgE-binding components (40, 71, 82 kDa), which were digested by simulated intestinal fluid and moderately digested by simulated gastric fluid, and the digestibility of allergens remained unchanged after thermal treatment. In conclusion, IgE-sensitization rate to com-mon whelk was 4.9% in allergy patients. IgE-immunoblotting demonstrated three IgE-binding components, which were degraded by digestive enzymes. Further studies are needed to evaluate the clinical significance of the sensitized patients to common whelk.
Keyword
MeSH Terms
-
Allergens/immunology
Animals
Comparative Study
Cookery
Digestion/*physiology
Food Handling/methods
Food Hypersensitivity/diagnosis/*epidemiology/*immunology/metabolism
Heat
Humans
Immunoglobulin E/*immunology/metabolism
Intestines/enzymology
Korea/epidemiology
*Mollusca
Research Support, Non-U.S. Gov't
Shellfish/*adverse effects
Skin Tests
Stomach/enzymology
Figure
Reference
-
1. Valentinsson D. Reproductive cycle and maternal effects on offspring size and number in the neogastropod Buccinum undatum (L). Marine Biol. 2002. 140:1139–1147.2. Carrillo T, Rodriguez de Castro F, Blanco C, Castillo R, Quiralte J, Cuevas M. Anaphylaxis due to limpet ingestion. Ann Allergy. 1994. 73:504–508.3. Morikawa A, Kato M, Tokuyama K, Kuroume T, Minoshima M, Iwata S. Anaphylaxis to grand keyhole limpet (abalone-like shell-fish) and abalone. Ann Allergy. 1990. 65:415–417.4. Lopata AL, Zinn C, Potter PC. Characteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae). J Allergy Clin Immunol. 1997. 100:642–648.
Article5. Amoroso S, Cocchiara R, Locorotondo G, Parlato A, Lampiasi N, Albeggiani G, Falagiani P, Geraci D. Antigens of Euparipha pisana (snail). I. Identification of allergens by means of in vivo and in vitro analysis. Int Arch Allergy Appl Immunol. 1988. 85:69–75.6. Nettis E, Pannofino A, Dambra P, Loria MP, Di Maggio G, Damiani E, Ferrannini A, Tursi A. IgE-mediated urticaria/angioedema after ingestion of mussels. Acta Derm Venereol. 2001. 81:62.7. Carrillo T, Castillo R, Caminero J, Cuevas M, Rodriguez JC, Acosta O, Rodriguez de Castro F. Squid hypersensitivity: a clinical and immunologic study. Ann Allergy. 1992. 68:483–487.8. Korea Rural and Economic Institute. Annual food demand and supply in 2001 [in Korean]. 2001. 129–147.9. Lee SK, Jee YK, Kim YK, Suh JH, Lee MH, Park HS. Identification of IgE binding components of Tetranychus urticae: species-specific and cross-reacting allergens with house dust mite [in Korean]. Korean J Asthma Allergy Clin Immunol. 2000. 20:879–886.10. Park HS. Allergy skin tests in clinical practice [in Korean]. J Asthma Allergy Clin Immunol. 2001. 21:115–119.11. Kim YK, Park HS, Kim HA, Lee MH, Choi JH, Kim SS, Lee SK, Nahm DH, Cho SH, Min KU, Kim YY. Two-spotted spider mite allergy: immunoglobulin E sensitization and characterization of allergenic components. Ann Allergy Asthma Immunol. 2002. 89:517–522.
Article12. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996. 14:1269–1273.
Article13. Yagami T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000. 106:752–762.
Article14. Tjärnö Marine Biological Laboratory, Stromstad, Sweden. Aquascope: Common whelk. accessed June 5, 2003. http://www.vattenkikaren.gu.se/fakta/arter/mollusca/prosobra/buccunda/buccu12e.html.15. Daul CB, Morgan JE, Lehrer SB. Hypersensitivity reactions to crustacea and mollusks. Clin Rev Allergy. 1993. 11:201–222.16. Choi JH, Yoon SH, Suh YJ, Suh CH, Nahm DH, Kim YK, Min KU, Park HS. Measurement of specific IgE to abalone (Haliotis discus hannai) and identification of IgE binding components [in Korean]. J Asthma Allergy Clin Immunol. 2003. 23:349–357.17. Miyazawa H, Fukamachi H, Inagaki Y, Reese G, Daul CB, Lehrer SB, Inouye S, Sakaguchi M. Identification of the first major allergen of a squid (Todarodes pacificus). J Allergy Clin Immunol. 1996. 98:948–953.
Article18. Smillie LB. Preparation and identification of alpha- and beta-tropomyosins. Methods Enzymol. 1982. 85:234–241.19. Leung PS, Chow WK, Duffey S, Kwan HS, Gershwin ME, Chu KH. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergen. J Allergy Clin Immunol. 1996. 98:954–961.
Article20. Asturias JA, Eraso E, Arilla MC, Gomez-Bayon N, Inacio F, Martinez A. Cloning, isolation, and IgE-binding properties of Helix aspersa (brown garden snail) tropomyosin. Int Arch Allergy Immunol. 2002. 128:90–96.21. Sidenius KE, Hallas TE, Poulsen LK, Mosbech H. Allergen cross-reactivity between house-dust mites and other invertebrates. Allergy. 2001. 56:723–733.
Article22. Diaz-Perales A, Blanco C, Sanchez-Monge R, Varela J, Carrillo T, Salcedo G. Analysis of avocado allergen (Prs a 1) IgE-binding peptides generated by simulated gastric fluid digestion. J Allergy Clin Immunol. 2003. 112:1002–1007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Common whelk (Buccinum undatum) allergy: Sensitization rate and its relationship with other food allergens
- Identification of the Major Allergen in the Shrimp (Metapenaeus Joyneri): Effects of Heating and Digestive Enzymes
- Chestnut as a Food Allergen: Identification of Major Allergens
- Clinical Features of Patients with Apple Allergy and Identification of IgE-Binding Components of Apple
- IgE Sensitization and Identification of IgE Binding Components of Soybean Allergen in Adult Allergy Patients