J Korean Med Sci.
2004 Dec;19(6):779-782. 10.3346/jkms.2004.19.6.779.
Phase Variation of Biofilm Formation in Staphylococcus aureus by IS 256 Insertion and Its Impact on the Capacity Adhering to Polyurethane Surface
- Affiliations
-
- 1Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul Korea. smkimkor@smc.samsung.co.kr
- 2Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul Korea.
- 3Asian-Pacific Research Foundation for Infectious Diseases (ARFID), Seoul Korea.
- 4Department of Microbiology, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1778556
- DOI: http://doi.org/10.3346/jkms.2004.19.6.779
Abstract
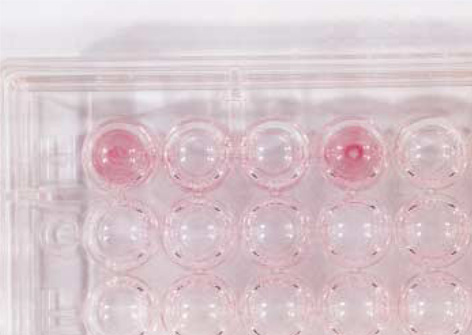
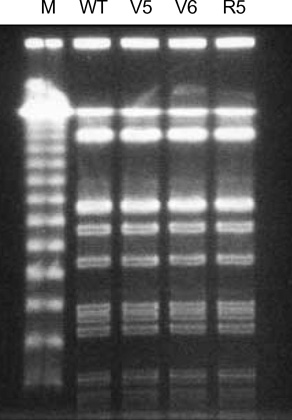
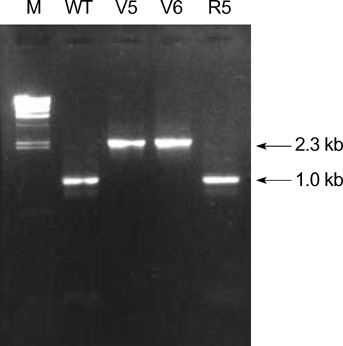
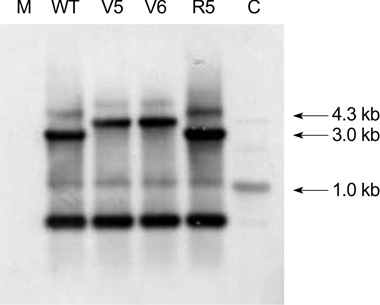
- While ica gene of Staphylococcus epidermidis is known to undergo phase variation by insertion of IS256, the phenomenon in Staphylococcus aureus has not been evaluated. Six biofilm-positive strains were tested for the presence of biofilm-nega-tive phase-variant strains by Congo red agar test. For potential phase-variant strains, pulsed-field gel electrophoresis was done to exclude the possibility of contamination. To investigate the mechanism of the biofilm-negative phase variation, PCR for each ica genes were done. Changes of ica genes detected by PCR were confirmed by southern hybridization, and their nucleotides were analyzed by DNA sequencing. Influence of ica genes and biofilm formation on capacity for adherence to biomedical material was evaluated by comparing the ability of adhering to polyurethane sur-face among a biofilm-negative phase-variant strain and its parent strain. A biofilm-negative phase-variant S. aureus strain was detected from 6 strains tested. icaC gene of the phase-variant strain was found to be inactivated by insertion of additional gene segment, IS256. The biofilm-negative phase-variant strain showed lower adher-ing capacity to polyurethane than its parent strain. This study shows that phase variation of ica gene occurs in S. aureus by insertion of IS256 also, and this biofilm-neg-ative phase variation reduces adhering capacity of the bacteria.
MeSH Terms
-
Bacterial Adhesion/*physiology
Biofilms/*growth & development
Cell Adhesion Molecules/genetics/*metabolism
Comparative Study
Equipment Contamination/prevention & control
Mutagenesis, Insertional/methods
Mutagenesis, Site-Directed/genetics
Phase Transition
Polysaccharides, Bacterial/genetics/*metabolism
*Polyurethanes
Species Specificity
Staphylococcus aureus/cytology/*physiology
Structure-Activity Relationship
Figure
Reference
-
1. Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996. 64:277–282.
Article2. Heilmann C, Hussain M, Peters G, Götz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol Microbiol. 1997. 24:1013–1024.3. Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996. 20:1083–1091.
Article4. Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996. 178:175–183.5. Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase polysaccharide intercellular adhesin. Infect Immun. 1994. 62:3244–3253.6. Gerke C, Kraft A, Süssmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyl-transferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998. 273:18586–18593.7. Christensen GD, Baddour LM, Simpson WA. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987. 55:2870–2877.
Article8. Ziebuhr W, Krimmer V, Rachid S, Lötzssner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis : evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS 256. Mol Microbiol. 1999. 32:345–356.9. Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999. 67:5427–5433.
Article10. Baselga R, Albizu I, De La Cruz M, Del Cacho E, Barberan M, Amorena B. Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun. 1993. 61:4857–4862.
Article11. Freeman D, Falkiner F, Keane C. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989. 42:872–874.
Article12. Goering RV, Winters MA. Rapid method for epidemiological evaluation of Gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol. 1992. 30:577–580.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Biofilm Formation on The Antimicrobial Susceptibility of Staphylococcus aureus
- Influence of Cell Surface Hydrophobicity on Adhesion and Biofilm Formation in Candida albicans and Several Bacterial Species
- Intramammary preparation of enrofloxacin hydrochloride-dihydrate for bovine mastitis (biofilm-forming Staphylococcus aureus)
- Association of biofilm production with colonization among clinical isolates of Acinetobacter baumannii
- Invasive potential of biofilm-forming Staphylococci bovine subclinical mastitis isolates