J Korean Med Sci.
2009 Jan;24(Suppl 1):S57-S62. 10.3346/jkms.2009.24.S1.S57.
The Effect of Lactic Acid Bacteria Isolates on the Urinary Tract Pathogens to Infants In Vitro
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Chung-Ang University, Seoul, Korea. ctrhslee@hotmail.com
- 2Department of Microbiology, College of Medicine, Chung-Ang University, Seoul, Korea.
- KMID: 1778141
- DOI: http://doi.org/10.3346/jkms.2009.24.S1.S57
Abstract
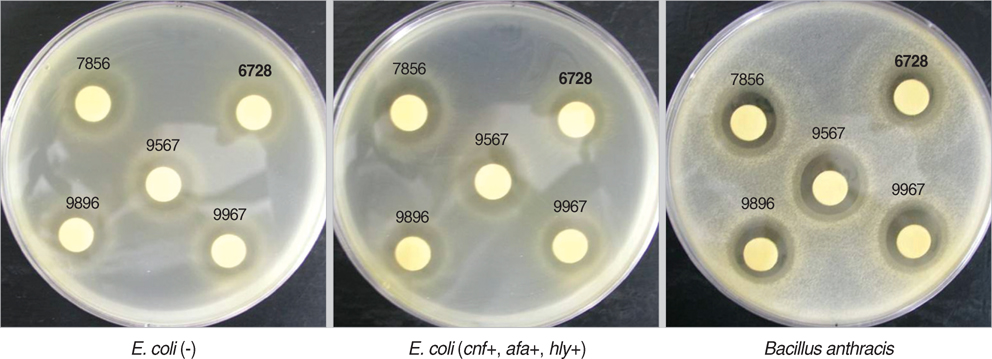
- Urinary tract infections are common clinical problems in children, even though lots of treatment strategies have been tried. Many studies of the application of probiotics for urinary tract infection in female adults exist, but there is a lack of studies in children. The aims of this study were to screen probiotic strains for inhibiting the uropathogens in vitro, to find candidates for in vivo study. Nine strains of E. coli were isolated from children with urinary tract infection and six uropathogens were obtained from Korean Colletion for Type Cultures and American Type Culture Collection. Also 135 lactic acid bacteria (LAB) strains were isolated from healthy children, and were identified through physiologic, biochemical methods, 16S rDNA PCR, and data analysis. And with agar disk diffusion assay technique the antimicrobial activities of these LAB strains against those uropathogens were examined. Three strains of separated LAB strains demonstrated major antimicrobial activity against all the uropathogens. In the agar disk diffusion assay technique, antimicrobial activities increased most in the 4th day culture broth with separated Lactobacillus. In summary, some LAB can be used as candidates to develop the probiotic microorganisms that inhibit uropathogens in children, and are expected to be applied to treatment and prevention of pediatric urinary tract infection.
MeSH Terms
-
Agar/chemistry
Anti-Infective Agents/pharmacology
Child
Culture Media/metabolism
Diffusion
Escherichia coli/*metabolism
Feces
Humans
Korea
Lactic Acid/*metabolism
Microbial Sensitivity Tests
Polymerase Chain Reaction
Probiotics/*metabolism
RNA, Ribosomal, 16S/metabolism
Urinary Tract Infections/*microbiology/therapy
Figure
Reference
-
1. Kunin CE. Urinary tract infections. 1979. 3rd ed. Philadelphia, PA: Lea & Febiger.2. Newman D. The treatment of cystitis by intravesical infections of lactic Bacillus cultures. Lancet. 1915. 14:330–332.3. Macfarlane GT, Cummings JH. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999. 318:999–1003.
Article4. Floch MH. Buchman A, editor. Prebiotics, probiotics and dietary fiber. Clinical nutrition: a guide for gastroenterologists. 2005. Thorofare, NJ: Slack Incorporated.5. Tannock GW. Probiotics and Prebiotics: Scientific Aspects. 2005. 1st ed. Norfolk, UK: Caister Academic Press;25–49.6. Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000. 95:1 Suppl. S11–S13.
Article7. Guarino A, Canani RB, Spagnuolo MI, Albano F, Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997. 25:516–519.
Article8. Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A, de Sousa JS, Sandhu B, Szajewska H, Weizman Z. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000. 30:54–60.
Article9. Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease : 4-year follow-up of a randomized placebo-controlled trial. Lancet. 2003. 361:1869–1871.10. Reid G, Bruce AW, Taylor M. Instillation of Lactobacillus and stimulation of indigenous organisms to prevent recurrence of urinary tract infections. Microecol Ther. 1995. 23:32–45.11. Lane S, Evermann J, Loge F, Call DR. Amplicon secondary structure prevents target hybridization to oligonucleotide microarrays. Biosens Bioelectron. 2004. 20:728–735.
Article12. Mombelli B, Gismondo MR. The use of probiotics in medical practice. Int J Antimicrob Agents. 2000. 16:531–536.
Article13. McGroarty JA, Reid G. Detection of a Lactobacillus substance which inhibits Escherichia coli. Can J Microbiol. 1988. 34:974–978.14. Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Envir Microbiol. 1999. 65:351–354.15. Lewenstein A, Frigerio G, Moroni M. Biological properties of SF68, a new approach for the treatment of diarrhoeal diseases. Curr Ther Res. 1979. 26:967–981.16. Salminen S, Deighton M. Lactic acid bacteria in the gut in normal and disordered states. Dig Dis. 1992. 10:227–238.
Article17. Reid G, Bruce AW. Selection of lactobacillus strains for urogenital probiotic applications. J Infect Dis. 2001. 183:Suppl 1. S77–S80.18. Gardiner GE, Heinemann C, Bruce AW, Beuerman D, Reid G. Persistence of Lactobacillus fermentum RC-14 and L. rhamnosus GR-1 but not L. rhamnosus GG in the human vagina as demonstrated by randomly amplified polymorphic DNA. Clin Diagn Lab Immunol. 2002. 9:92–96.19. Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, Kitamura T, Kawabe K, Kotake T, Kuroda M, Naito S, Koga H, Saito Y, Nomata K, Kitagawa M, Aso Y. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int. 2002. 68:273–280.
Article20. Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002. 68:3841–3847.
Article21. Reid G, Cook RL, Bruce AW. Examination of strains of lactobacilli for properties which may influence bacterial interference in the urinary tract. J Urol. 1987. 138:330–335.22. Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000. 130:2S Suppl. S403–S409.
Article23. McFarland LV. Normal flora: diversity and functions. Microb Ecol Health Dis. 2000. 12:193–207.
Article24. Weinberg ED, Weinberg GA. The role of iron in infection. Curr Opin Infect Dis. 1995. 8:164–169.
Article25. Mackay AD, Taylor MB, Kibbler CC, Hamilton-Miller JM. Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect. 1999. 5:290–292.
Article26. Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J Clin Microbiol. 1998. 36:325–326.27. Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, Koskela M. Liver abscess due to a Lactobacillus rhamnosus strain in distinguishable from L. rhamnosus strain GG. Clin Infect Dis. 1999. 28:1159–1160.28. Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE, Mattila-Sandholm T. Demonstration of safety of probiotics-a review. Int J Food Microbiol. 1998. 44:93–106.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifungal Activity of Lactic Acid Bacteria Isolated from Kimchi Against Aspergillus fumigatus
- In vivo antitumor effects of lactic acid bacteria on sarcoma 180 and mouse lewis lung carcinoma
- Inhibitory effects of Enterococcus faecium isolated from Korean infants on oral pathogens
- A survey of research papers on the health benefits of kimchi and kimchi lactic acid bacteria
- Differences in Urine Microbiome of Acute Cystitis and Chronic Recurrent Cystitis in Women