J Korean Med Sci.
2010 Mar;25(3):492-495. 10.3346/jkms.2010.25.3.492.
Aloe-induced Toxic Hepatitis
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Hallym University, Chuncheon, Korea. djkim@hallym.ac.kr
- 2Department of Pathology, College of Medicine, Hallym University, Chuncheon, Korea.
- KMID: 1778051
- DOI: http://doi.org/10.3346/jkms.2010.25.3.492
Abstract
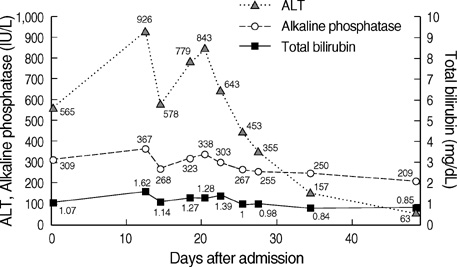
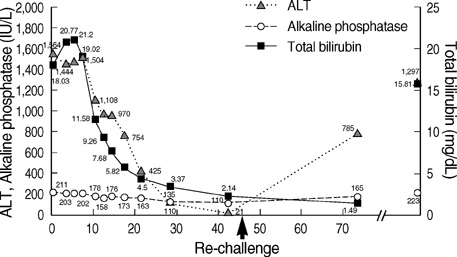
- Aloe has been widely used in phytomedicine. Phytomedicine describes aloe as a herb which has anti-inflammatory, anti-proliferative, anti-aging effects. In recent years several cases of aloe-induced hepatotoxicity were reported. But its pharmacokinetics and toxicity are poorly described in the literature. Here we report three cases with aloe-induced toxic hepatitis. A 57-yr-old woman, a 62-yr-old woman and a 55-yr-old woman were admitted to the hospital for acute hepatitis. They had taken aloe preparation for months. Their clinical manifestation, laboratory findings and histologic findings met diagnostic criteria (RUCAM scale) of toxic hepatitis. Upon discontinuation of the oral aloe preparations, liver enzymes returned to normal level. Aloe should be considered as a causative agent in hepatotoxicity.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Acute Toxic Hepatitis Caused by an Aloe Vera Preparation in a Young Patient: A Case Report with a Literature Review
Jeonghun Lee, Mi Sun Lee, Kwan Woo Nam
Korean J Gastroenterol. 2014;64(1):54-58. doi: 10.4166/kjg.2014.64.1.54.
Reference
-
1. Food and Drug Statistical Yearbook. Korea Food and Drug Administration. 2006. 08. accessed 2008 0ct 27. Available at: URL: http://www.kfda.go.kr/open_content/main/main.php.2. Yoo TW, Kim BI, Kim JB, Kim DJ, Kim JW, Baik SK, Kim KS, Cheon GJ. The survey for the actual condition of drug medication and development of health care cost associated with toxic liver injury in Korean: a multicenter study for the detection and the development of nationwide reporting system of toxic liver injury. Korean J Hepatol. 2007. 13:34–43.3. Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis induced by an aloe vera preparation: a case report. World J Gastroenterol. 2005. 11:303–304.
Article4. Kanat O, Ozet A, Ataergin S. Aloe vera-induced acute toxic hepatitis in a healthy young man. Eur J Intern Med. 2006. 17:589.
Article5. Bottenberg MM, Wall GC, Harvey RL, Habib S. Oral aloe vera-induced hepatitis. Ann Pharmacother. 2007. 41:1740–1743.
Article6. Schoepfer AM, Engel A, Fattinger K, Marbet UA, Criblez D, Reichen J, Zimmermann A, Oneta CM. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from herbalife products. J Hepatol. 2007. 47:521–526.
Article7. Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990. 11:272–276.8. Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005. 43:901–910.
Article9. Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999. 49:823–828.10. Jang DD, Cho JC, Choi KS, Kil HI. Studies of the effectiveness and toxicity of aloe vera gel. The report of National Institute of Safety Research. 1994. 7:225–233.11. Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006. 354:731–739.
Article12. Chae HB. Clinical features and diagnosis of drug induced liver injury. Korean J Hepatol. 2004. 10:Suppl1. 7–18.13. Kang DY. Pathologic features of toxic and drug induced liver injury. Korean J Hepatol. 2004. 10:Suppl1. 19–29.14. Morrow DM, Rapaport MJ, Strick RA. Hypersensitivity to aloe. Arch Dermatol. 1980. 116:1064–1065.
Article15. Lee EG, Kwon SH, Kim SH, Myung SJ, Choi JW, Kim YJ, Ahn Y. A case of hypersensitivity associated with oral aloe agent. J Asthma Allergy Clin Immunol. 2003. 23:833–836.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Toxic Hepatitis Caused by an Aloe Vera Preparation in a Young Patient: A Case Report with a Literature Review
- Acute Toxic Hepatitis: RUCAM Application to Drug-induced Liver Injury and Its Limitations
- A case of hypersensitivity associated with oral aloe agent
- Amanita virosa induced toxic hepatitis: report of three cases
- A Case of Toxic Erythema, Toxic Hepatitis and Exfoliative Deratitis due to Trichloroethylene