J Korean Med Sci.
2011 May;26(5):665-674. 10.3346/jkms.2011.26.5.665.
Altered Brain Activation in Ventral Frontal-Striatal Regions Following a 16-week Pharmacotherapy in Unmedicated Obsessive-Compulsive Disorder
- Affiliations
-
- 1Interdisciplinary Program in Neuroscience, Seoul National University, Seoul, Korea. kwonjs@snu.ac.kr
- 2Clinical Cognitive Neuroscience Center, Neuroscience Institute, SNU-MRC, Seoul, Korea.
- 3Department of Psychiatry, Seoul National University College of Medicine & Hospital, Seoul, Korea.
- 4Department of Radiology, National Medical Center, Seoul, Korea.
- 5Brain and Cognitive Science-WCU Program, Seoul National University College of Natural Science, Seoul, Korea.
- KMID: 1777867
- DOI: http://doi.org/10.3346/jkms.2011.26.5.665
Abstract
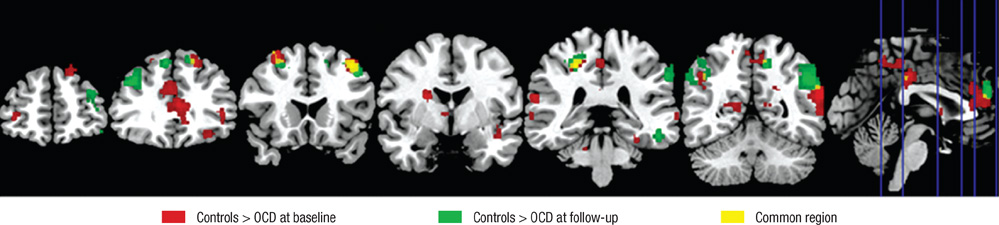
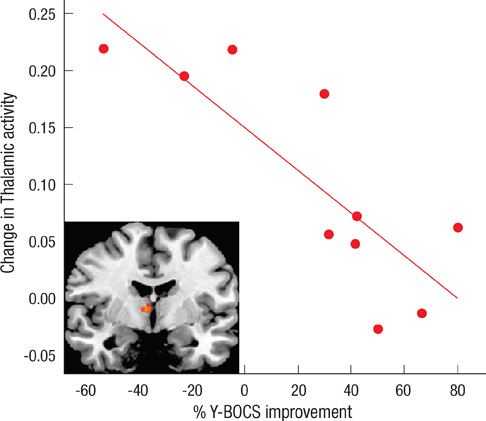
- Recent studies have reported that cognitive inflexibility associated with impairments in a frontal-striatal circuit and parietal region is a core cognitive deficit of obsessive-compulsive disorder (OCD). However, few studies have examined progressive changes in these regions following clinical improvement in obsessive-compulsive symptoms. To determine if treatment changes the aberrant activation pattern associated with task switching in OCD, we examined the activation patterns in brain areas after treatment. The study was conducted on 10 unmedicated OCD patients and 20 matched controls using event-related functional magnetic resonance imaging. Treatment improved the clinical symptoms measured by the Yale-Brown Obsessive Compulsive Scale and behavioral flexibility indicated by the switching cost. At baseline, OCD showed significantly less activation in the dorsal and ventral frontal-striatal circuit and parietal regions under the task-switch minus task-repeat condition compared with controls. After treatment, the neural responses in the ventral frontal-striatal circuit in OCD were partially normalized, whereas the activation deficit in dorsal frontoparietal regions that mediate shifting attention or behavioral flexibility persisted. It is suggested that altered brain activation in ventral frontal-striatal regions in OCD patients is associated with their cognitive flexibility and changes in these regions may underlie the pathophysiology of OCD.
Keyword
MeSH Terms
Figure
Reference
-
1. Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000. 28:343–347.2. Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008. 32:525–549.3. Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008. 131:155–164.4. Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Aitken M, Craig K, Owen AM, Bullmore ET, Robbins TW, Sahakian BJ. Orbitofrontal dysfunction in patients with obsessive-compulsive disorder and their unaffected relatives. Science. 2008. 321:421–422.5. Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry. 2006. 63:1225–1236.6. Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Rev Neurother. 2009. 9:255–269.7. Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatr Clin North Am. 2006. 29:391–410.8. van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch Gen Psychiatry. 2005. 62:301–309.9. Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am. 2000. 23:563–586.10. Lázaro L, Caldú X, Junqué C, Bargalló N, Andrés S, Morer A, Castro-Fornieles J. Cerebral activation in children and adolescents with obsessive-compulsive disorder before and after treatment: a functional MRI study. J Psychiatr Res. 2008. 42:1051–1059.11. Nabeyama M, Nakagawa A, Yoshiura T, Nakao T, Nakatani E, Togao O, Yoshizato C, Yoshioka K, Tomita M, Kanba S. Functional MRI study of brain activation alterations in patients with obsessive-compulsive disorder after symptom improvement. Psychiatry Res. 2008. 163:236–247.12. Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, Kudoh A, Tada K, Yoshioka K, Kawamoto M, Togao O, Kanba S. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005. 57:901–910.13. Hansen ES, Hasselbalch S, Law I, Bolwig TG. The caudate nucleus in obsessive-compulsive disorder. Reduced metabolism following treatment with paroxetine: a PET study. Int J Neuropsychopharmacol. 2002. 5:1–10.14. Kang DH, Kwon JS, Kim JJ, Youn T, Park HJ, Kim MS, Lee DS, Lee MC. Brain glucose metabolic changes associated with neuropsychological improvements after 4 months of treatment in patients with obsessive-compulsive disorder. Acta Psychiatr Scand. 2003. 107:291–297.15. Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N, Ho MK, Huang SC, Wu HM, Baxter LR Jr. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002. 59:250–261.16. Saxena S, Brody AL, Maidment KM, Dunkin JJ, Colgan M, Alborzian S, Phelps ME, Baxter LR Jr. Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology. 1999. 21:683–693.17. Nielen MM, Den Boer JA. Neuropsychological performance of OCD patients before and after treatment with fluoxetine: evidence for persistent cognitive deficits. Psychol Med. 2003. 33:917–925.18. Henry JD. A meta-analytic review of Wisconsin Card Sorting Test and verbal fluency performance in obsessive-compulsive disorder. Cogn Neuropsychiatry. 2006. 11:156–176.19. Kim MS, Park SJ, Shin MS, Kwon JS. Neuropsychological profile in patients with obsessive-compulsive disorder over a period of 4-month treatment. J Psychiatr Res. 2002. 36:257–265.20. Roh KS, Shin MS, Kim MS, Ha TH, Shin YW, Lee KJ, Kwon JS. Persistent cognitive dysfunction in patients with obsessive-compulsive disorder: a naturalistic study. Psychiatry Clin Neurosci. 2005. 59:539–545.21. Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 1999. 156:1409–1416.22. Monsell S. Task switching. Trends Cogn Sci. 2003. 7:134–140.23. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009. 195:393–402.24. Ho Pian KL, van Megen HJ, Ramsey NF, Mandl R, van Rijk PP, Wynne HJ, Westenberg HG. Decreased thalamic blood flow in obsessive-compulsive disorder patients responding to fluvoxamine. Psychiatry Res. 2005. 138:89–97.25. Yoo SY, Jang JH, Shin YW, Kim DJ, Park HJ, Moon WJ, Chung EC, Lee JM, Kim IY, Kim SI, Kwon JS. White matter abnormalities in drug-naive patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr Scand. 2007. 116:211–219.26. Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, Lyoo IK, Cho MJ, Lee MC. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res. 2003. 122:37–47.27. Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008. 13:829833–857.28. Zitterl W, Aigner M, Stompe T, Zitterl-Eglseer K, Gutierrez-Lobos K, Wenzel T, Zettinig G, Hornik K, Pirker W, Thau K. Changes in thalamus-hypothalamus serotonin transporter availability during clomipramine administration in patients with obsessive-compulsive disorder. Neuropsychopharmacology. 2008. 33:3126–3134.29. Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA. 2000. 97:13448–13453.30. Jang JH, Kwon JS, Jang DP, Moon WJ, Lee JM, Ha TH, Chung EC, Kim IY, Kim SI. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naive patients with obsessive-compulsive disorder. Am J Psychiatry. 2006. 163:1202–1207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Onset Obsessive Compulsive Disorder Following High Frequency Repetitive Transcranial Magnetic Stimulation over Left Dorsolateral Prefrontal Cortex for Treatment of Negative Symptoms in a Patient with Schizophrenia
- Obsessive-Compulsive Disorder
- Functional Connectivity of the Striatum as a Neural Correlate of Symptom Severity in Patient with Obsessive-Compulsive Disorder
- Aripiprazole Improved Obsessive Compulsive Symptoms in Asperger's Disorder
- Pharmacological Treatment for Body Dysmorphic Disorder