J Korean Med Sci.
2011 Aug;26(8):1023-1030. 10.3346/jkms.2011.26.8.1023.
Clinicopathologic Study on Combined Hepatocellular Carcinoma and Cholangiocarcinoma: with Emphasis on the Intermediate Cell Morphology
- Affiliations
-
- 1Department of Pathology, Chonbuk National University Medical School, Institute for Medical Sciences, Research Institute of Clinical Medicine, Jeonju, Korea. mws@chonbuk.ac.kr
- 2Department of Preventive Medicine, Chonbuk National University Medical School, Institute for Medical Sciences, Research Institute of Clinical Medicine, Jeonju, Korea.
- 3Department of Surgery, Chonbuk National University Medical School, Institute for Medical Sciences, Research Institute of Clinical Medicine, Jeonju, Korea.
- 4Research Institute for Endocrine Sciences, Jeonju, Korea.
- KMID: 1777840
- DOI: http://doi.org/10.3346/jkms.2011.26.8.1023
Abstract
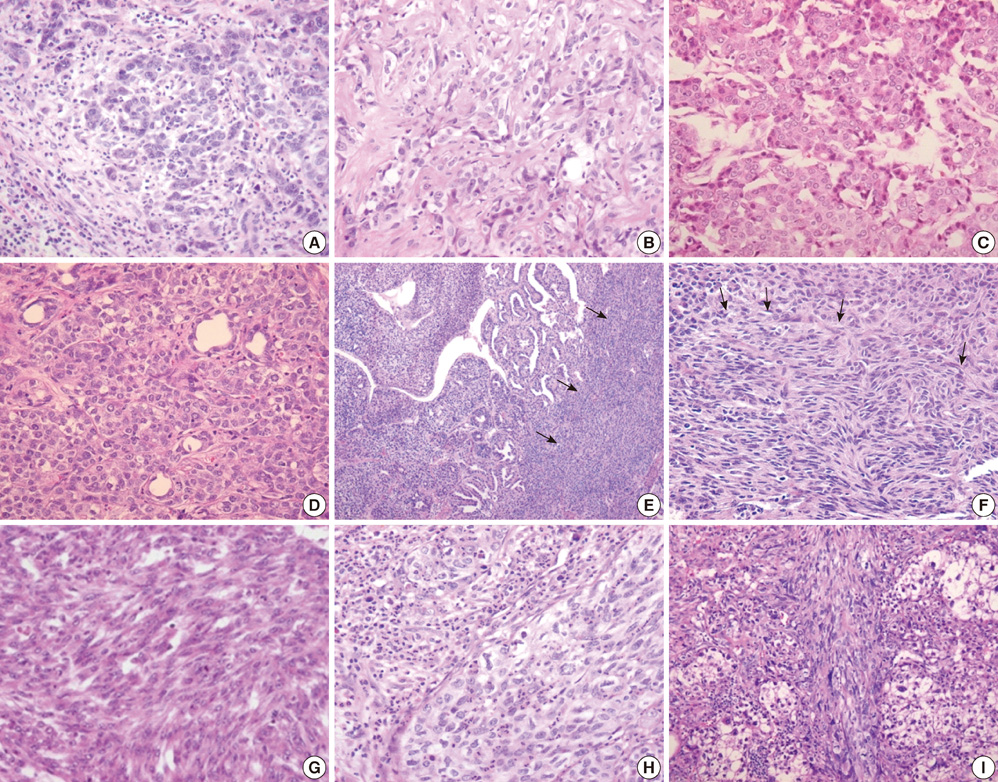
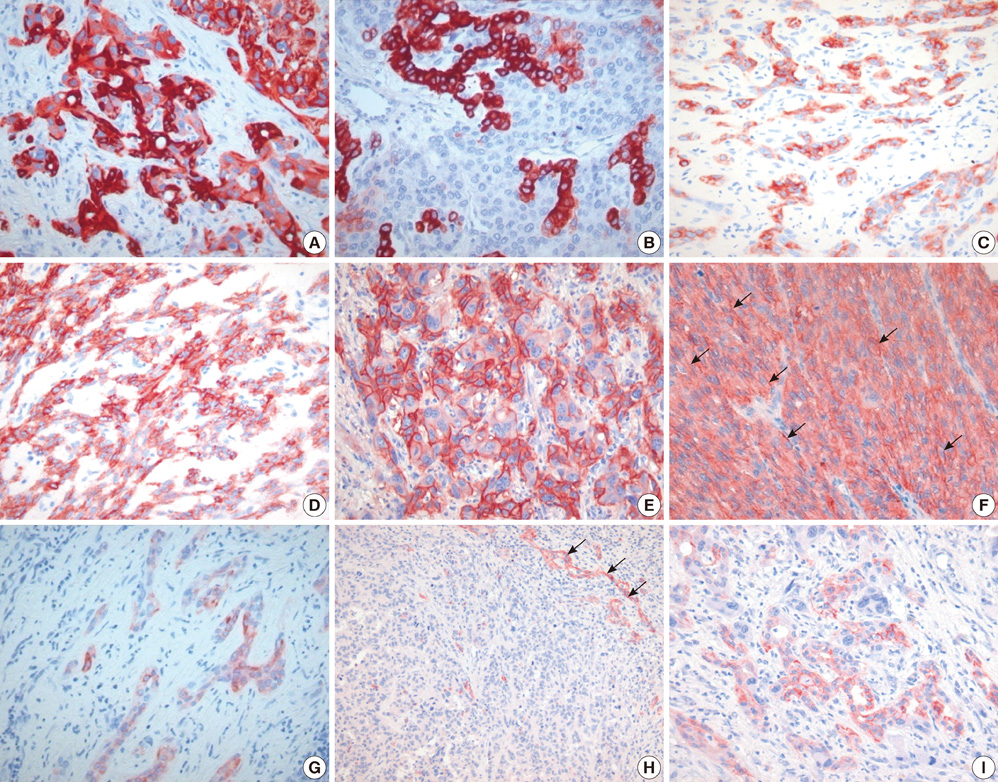
- Combined hepatocellular carcinoma and cholangiocarcinoma (combined HCC-CC) is a rare subtype of primary liver cancer. We investigated the histopathologic features of transitional or intermediate areas in 21 combined HCC-CCs and immunophenotypes using different hepatic progenitor cell markers (CK7, CK19, c-kit, NCAM, and EpCAM). Major histologic findings of transitional or intermediate areas of 21 combined HCC-CCs included strands/trabeculae of small, uniform, oval-shaped cells with scant cytoplasm and hyperchromatic nuclei embedded within an abundant stroma, small cells with an antler-like anastomosing pattern, and solid nests of intermediate hepatocyte-like cells surrounded by small cells in periphery, in order of frequency. The intermediate area of one tumor was composed predominantly of spindle cells arranged in short fascicles. Immunophenotype of tumor cells with intermediate morphology suggested a progenitor cell origin for this tumor. Clinical findings of combined HCC-CC showed a closer resemblance with those of HCC than those of CC. In univariate analysis, tumor size, TNM stage, and serum alpha-fetoprotein levels showed a significant association with poor patient survival. Serum alpha-fetoprotein level was an independent prognostic indicator in multivariate analysis. In conclusion, an awareness of the clinicopathologic features, specifically the various morphologic features of intermediate areas in this tumor, is essential for prevention of potential misdiagnosis as another tumor.
Keyword
MeSH Terms
-
Adult
Aged
Antigens, Neoplasm/metabolism
Carcinoma, Hepatocellular/*pathology
Cell Adhesion Molecules/metabolism
Cholangiocarcinoma/*pathology
Female
Humans
Immunophenotyping
Keratin-19/metabolism
Keratin-7/metabolism
Liver Neoplasms/*pathology
Male
Middle Aged
Neural Cell Adhesion Molecules/metabolism
Prognosis
Proto-Oncogene Proteins c-kit/metabolism
alpha-Fetoproteins/analysis
Figure
Cited by 1 articles
-
Clinicopathological characteristics and prognostic factors in combined hepatocellular carcinoma and cholangiocarcinoma
Sang Eun Park, Sung Ha Lee, Jae Do Yang, Hong Pil Hwang, Si Eun Hwang, Hee Chul Yu, Woo Sung Moon, Baik Hwan Cho
Korean J Hepatobiliary Pancreat Surg. 2013;17(4):152-156. doi: 10.14701/kjhbps.2013.17.4.152.
Reference
-
1. Theise ND, Nakashima O, Park YN, Nakanuma Y. Bosman FT, Garneiro F, Hruban RH, Theise ND, editors. Combined hepatocellular-cholangiocarcinoma. WHO classification of tumours of the digestive system. 2010. 4th ed. Lyon: IARC Press;225–227.2. Kojiro M. Kojiro M, editor. Combined hepatocellular carcinoma and cholaniocarcinoma. Pathology of hepatocellular carcinoma. 2006. 1st ed. Blackwell;105–115.3. Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008. 52:224–232.4. Kim H, Park C, Han KH, Choi J, Kim YB, Kim JK, Park YN. Primary liver carcinoma of intermediate (hepatocyte-cholangiocyte) phenotype. J Hepatol. 2004. 40:298–304.5. Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003. 43:263–271.6. Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W, Kulik M, Sherwood S, Tallheden T, Cheng N, Furth ME, Reid LM. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007. 204:1973–1987.7. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 2010. 7th ed. New York: Springer.8. Sasaki M, Tsuneyama K, Ishikawa A, Nakanuma Y. Intrahepatic cholangiocarcinoma in cirrhosis presents granulocyte and granulocyte-macrophage colony-stimulating factor. Hum Pathol. 2003. 34:1337–1344.9. Li YW, Qui SJ, Fan J, Zhou J, Gao Q, Xiao YS, Xu YF. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011. 54:497–505.10. Joshita S, Nakazawa K, Koike S, Kamijo A, Matsubayashi K, Miyabayashi H, Furuta K, Kitano K, Yoshizawa K, Tanaka E. A case of granulocyte-colony stimulation factor-producing hepatocellular carcinoma confirmed by immunohistochemistry. J Korean Med Sci. 2010. 25:476–480.11. Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol. 2002. 13:389–396.12. Libbrecht L. Hepatic progenitor cells in human liver tumor development. World J Gastroenterol. 2006. 12:6261–6265.13. Tickoo SK, Zee SY, Obiekwe S, Xiao H, Koea J, Robiou C, Blumgart LH, Jarnagin W, Ladanyi M, Klimstra DS. Combined hepatocellular-cholangiocarcinoma: a histopathologic, immunohistochemical, and in situ hybridization study. Am J Surg Pathol. 2002. 26:989–997.14. Tanaka S, Yamamoto T, Tanaka H, Kodai S, Ogawa M, Ichikawa T, Hai S, Sakabe K, Uenishi T, Shuto T, Kubo S. Potentiality of combined hepatocellular and intrahepatic cholangiocellular carcinoma originating from a hepatic precursor cell: immunohistochemical evidence. Hepatol Res. 2005. 32:52–57.15. Koh KC, Lee H, Choi MS, Lee JH, Paik SW, Yoo BC, Rhee JC, Cho JW, Park CK, Kim HJ. Clinicopathologic features and prognosis of combined hepatocellular cholangiocarcinoma. Am J Surg. 2005. 189:120–125.16. Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002. 94:2040–2046.17. Portolani N, Baiocchi GL, Coniglio A, Piardi T, Grazioli L, Benetti A, Ferrari Bravo A, Giulini SM. Intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma: a Western experience. Ann Surg Oncol. 2008. 15:1880–1890.18. Yano Y, Yamamoto J, Kosuge T, Sakamoto Y, Yamasaki S, Shimada K, Ojima H, Sakamoto M, Takayama T, Makuuchi M. Combined hepatocellular and cholangiocarcinoma: a clinicopathologic study of 26 resected cases. Jpn J Clin Oncol. 2003. 33:283–287.19. Zuo HQ, Yan LN, Zeng Y, Yang JY, Luo HZ, Liu JW, Zhou LX. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2007. 6:161–165.20. Lee JH, Chung GE, Yu SJ, Hwang SY, Kim JS, Kim HY, Yoon JH, Lee HS, Yi NJ, Suh KS, Lee KU, Jang JJ, Kim YJ. Long-term prognosis of combined hepatocellular and cholangiocarcinoma after curative resection comparison with hepatocellular carcinoma and cholangiocarcinoma. J Clin Gastroenterol. 2011. 45:69–75.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Hepatocellular-cholangiocarcinoma
- Combined Hepatocellular-Cholangiocarcinoma: Recent Progress in Pathology and Classification
- Combined Hepatocellular-cholangiocarcinoma
- Epidemiological Observation of Clonorchis sinensis Infection in Connection with Primary Carcinoma of the Liver
- Intermediate hepatic carcinoma mimicking intrahepatic cholangiocarcinoma: A case report