Yonsei Med J.
2013 Jan;54(1):92-100. 10.3349/ymj.2013.54.1.92.
Glioma Stem Cell-Targeted Dendritic Cells as a Tumor Vaccine Against Malignant Glioma
- Affiliations
-
- 1Department of Neurosurgery, Renmin Hospital of Wuhan University, Wuhan, China. chenqx666@sohu.com
- KMID: 1776923
- DOI: http://doi.org/10.3349/ymj.2013.54.1.92
Abstract
- PURPOSE
Cancer stem cells have recently been thought to be closely related to tumor development and reoccurrence. It may be a promising way to cure malignant glioma by using glioma stem cell-targeted dendritic cells as a tumor vaccine. In this study, we explored whether pulsing dendritic cells with antigens of glioma stem cells was a potent way to induce specific cytotoxic T lymphocytes and anti-tumor immunity.
MATERIALS AND METHODS
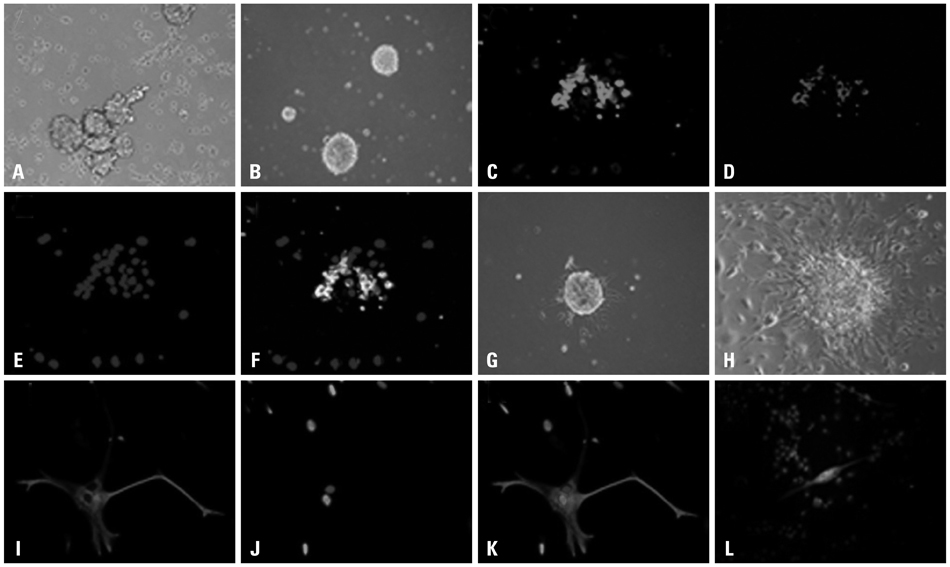
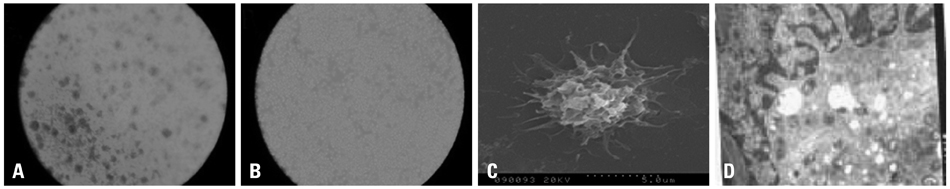
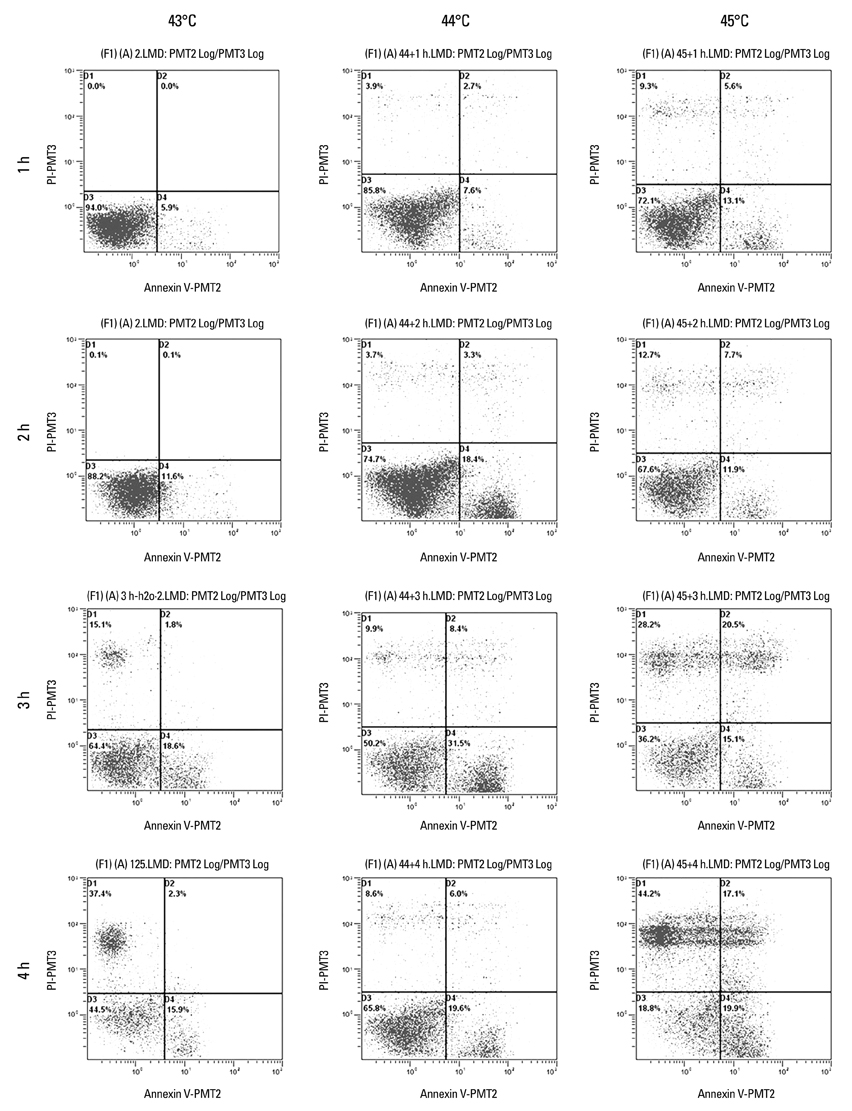
Cancer stem cells were cultured from glioma cell line U251. Lysate of glioma stem cells was obtained by the repeated freezing and thawing method. Dendritic cells (DCs) were induced and cultured from the murine bone marrow cells, the biological characteristics were detected by electron microscope and flow cytometry. The DC vaccine was obtained by mixing DCs with lysate of glioma stem cells. The DC vaccine was charactirizated through the mixed lymphocyte responses and cell killing experiment in vitro. Level of interferon-gamma (IFN-gamma) in the supernatant was checked by ELISA.
RESULTS
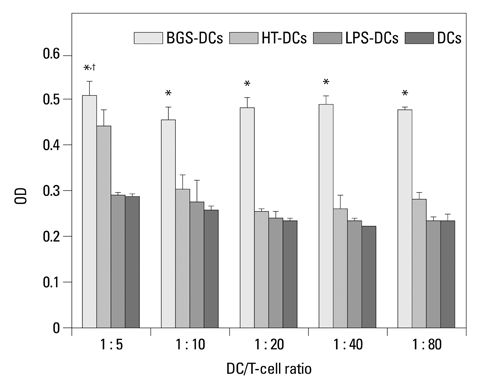
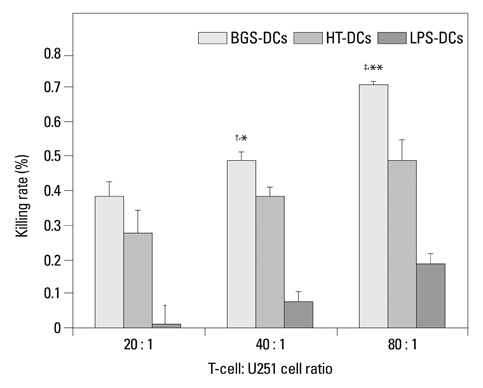
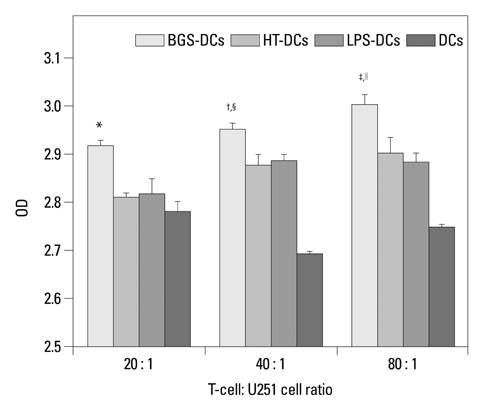
After stimulation of lysate of glioma stem cell, expression of surface molecules of DC was up-regulated, including CD80, CD86, CD11C and MHC-II. DCs pulsed with lysate of glioma stem cells were more effective than the control group in stimulating original glioma cells-specific cytotoxic T lymphocytes responses, killing glioma cells and boosting the secretion of IFN-gamma in vitro.
CONCLUSION
The results demonstrated DCs loaded with antigens derived from glioma stem cells can effectively stimulate naive T cells to form specific cytotoxic T cells, kill glioma cells cultured in vitro.
Keyword
MeSH Terms
-
Animals
Antigens, Neoplasm/immunology
Apoptosis
Brain Neoplasms/*therapy
Cancer Vaccines/*therapeutic use
Cell Line, Tumor
Cell Proliferation
Dendritic Cells/*cytology
Enzyme-Linked Immunosorbent Assay
Flow Cytometry
Glioma/*therapy
Humans
Interferon-gamma/metabolism
Male
Mice
Mice, Inbred C57BL
Neoplasm Transplantation
Neoplastic Stem Cells/*cytology
T-Lymphocytes, Cytotoxic/immunology
Antigens, Neoplasm
Cancer Vaccines
Interferon-gamma
Figure
Reference
-
1. Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009. 472:323–342.
Article2. Fecci PE, Mitchell DA, Archer GE, Morse MA, Lyerly HK, Bigner DD, et al. The history, evolution, and clinical use of dendritic cell-based immunization strategies in the therapy of brain tumors. J Neurooncol. 2003. 64:161–176.
Article3. Wheeler CJ, Black KL. Dendritic cell vaccines and obstacles to beneficial immunity in glioma patients. Curr Opin Mol Ther. 2005. 7:35–47.4. Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008. 12:67–96.5. Yao XH, Ping YF, Bian XW. Contribution of cancer stem cells to tumor vasculogenic mimicry. Protein Cell. 2011. 2:266–272.
Article6. Inagaki A, Soeda A, Oka N, Kitajima H, Nakagawa J, Motohashi T, et al. Long-term maintenance of brain tumor stem cell properties under at non-adherent and adherent culture conditions. Biochem Biophys Res Commun. 2007. 361:586–592.
Article7. Pellegatta S, Poliani PL, Corno D, Menghi F, Ghielmetti F, Suarez-Merino B, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006. 66:10247–10252.
Article8. Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001. 294:2186–2189.
Article9. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992. 176:1693–1702.
Article10. Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997. 186:1177–1182.
Article11. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007. 449:419–426.
Article12. Gu JH, Li G. Dendritic cell-based immunotherapy for malignant glioma. Neurosci Bull. 2008. 24:39–44.13. Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. J Immunol. 2004. 172:7341–7349.
Article14. Sagar J, Chaib B, Sales K, Winslet M, Seifalian A. Role of stem cells in cancer therapy and cancer stem cells: a review. Cancer Cell Int. 2007. 7:9.
Article15. Soltanian S, Matin MM. Cancer stem cells and cancer therapy. Tumour Biol. 2011. 32:425–440.
Article16. Dey M, Ulasov IV, Tyler MA, Sonabend AM, Lesniak MS. Cancer stem cells: the final frontier for glioma virotherapy. Stem Cell Rev. 2011. 7:119–129.
Article17. Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003. 63:5821–5828.18. Rogers LR, Wicha M. Rajasekhar VK, Vemuri MC, editors. Therapeutic approaches to target cancer stem cells. Regulatory Networks in Stem Cells. 2009. New York, NY, USA: Humana Press;545–560.
Article19. Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003. 100:15178–15183.
Article20. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004. 432:396–401.
Article21. Tabatabai G, Weller M. Glioblastoma stem cells. Cell Tissue Res. 2011. 343:459–465.
Article22. Lathia JD, Venere M, Rao MS, Rich JN. Seeing is believing: are cancer stem cells the Loch Ness monster of tumor biology? Stem Cell Rev. 2011. 7:227–237.
Article23. Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E, et al. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 2008. 3:e1936.
Article24. Prestegarden L, Svendsen A, Wang J, Sleire L, Skaftnesmo KO, Bjerkvig R, et al. Glioma cell populations grouped by different cell type markers drive brain tumor growth. Cancer Res. 2010. 70:4274–4279.
Article25. Bernard DJ, Courjal F, Maurizis JC, Bignon YJ, Chollet P, Plagne R. Effect of epidermal growth factor in HLA class I and class II transcription and protein expression in human breast adenocarcinoma cell lines. Br J Cancer. 1992. 66:88–92.
Article26. Sun L, Carpenter G. Epidermal growth factor activation of NF-kappaB is mediated through IkappaBalpha degradation and intracellular free calcium. Oncogene. 1998. 16:2095–2102.
Article27. Zhao J, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. A cell type-specific enhancer in the human B7.1 gene regulated by NF-kappaB. J Exp Med. 1996. 183:777–789.
Article28. Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. T suppressor lymphocytes inhibit NF-kappa B-mediated transcription of CD86 gene in APC. J Immunol. 1999. 163:6386–6392.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Immunotherapeutic Approaches for Malignant Gliomas
- Cancer Stem Cells in Brain Tumors and Their Lineage Hierarchy
- Molecular Culprits Generating Brain Tumor Stem Cells
- Cytotoxicities of Tumor-specific T Lymphocytes Primed by Glioma Apoptotic Body-or Glioma Cell Lysate-pulsed Dendritic Cells
- Brain Tumor Stem Cells as Therapeutic Targets in Models of Glioma