Korean Circ J.
2011 Jun;41(6):287-295. 10.4070/kcj.2011.41.6.287.
Hemorheology and Microvascular Disorders
- Affiliations
-
- 1Department of Mechanical Engineering and Mechanics, Drexel University Philadelphia, PA, USA. choyi@drexel.edu
- 2Rheovector LLC, Pennsauken, NJ, USA.
- KMID: 1776183
- DOI: http://doi.org/10.4070/kcj.2011.41.6.287
Abstract
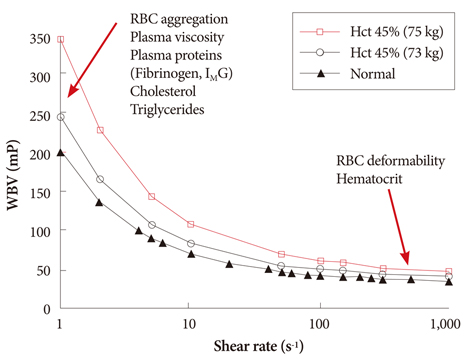
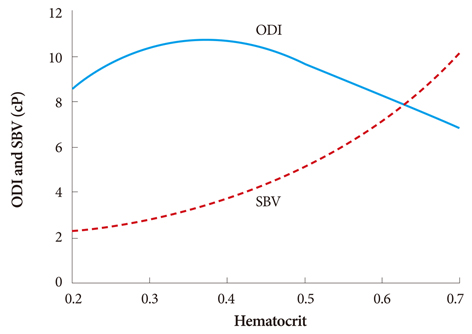
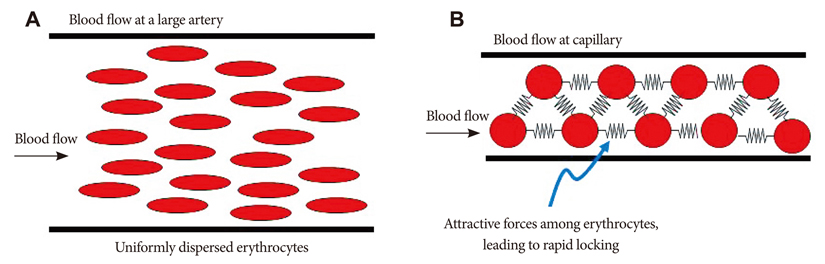
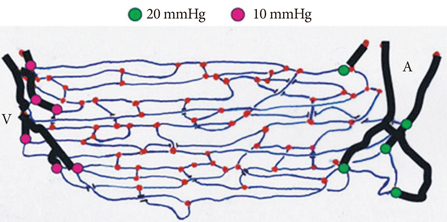
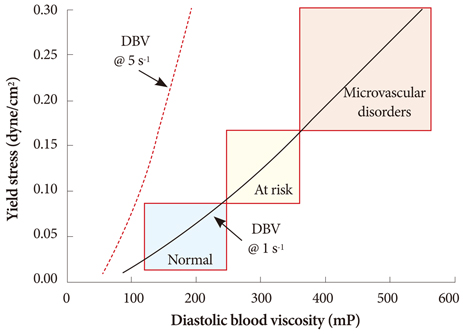
- The present review presents basic concepts of blood rheology related to vascular diseases. Blood flow in large arteries is dominated by inertial forces exhibited at high flow velocities, while viscous forces (i.e., blood rheology) play an almost negligible role. When high flow velocity is compromised by sudden deceleration as at a bifurcation, endothelial cell dysfunction can occur along the outer wall of the bifurcation, initiating inflammatory gene expression and, through mechanotransduction, the cascade of events associated with atherosclerosis. In sharp contrast, the flow of blood in microvessels is dominated by viscous shear forces since the inertial forces are negligible due to low flow velocities. Shear stress is a critical parameter in microvascular flow, and a force-balance approach is proposed for determining microvascular shear stress, accounting for the low Reynolds numbers and the dominance of viscous forces over inertial forces. Accordingly, when the attractive forces between erythrocytes (represented by the yield stress of blood) are greater than the shear force produced by microvascular flow, tissue perfusion itself cannot be sustained, leading to capillary loss. The yield stress parameter is presented as a diagnostic candidate for future clinical research, specifically, as a fluid dynamic biomarker for microvascular disorders. The relation between the yield stress and diastolic blood viscosity (DBV) is described using the Casson model for viscosity, from which one may be able determine thresholds of DBV where the risk of microvascular disorders is high.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Establishing Reference Intervals of Whole Blood Viscosity in a Korean Population Using a Cone-Plate Viscometer
Mikyoung Park, Hanah Kim, Hee-Won Moon, Mina Hur, Yeo-Min Yun
Lab Med Online. 2021;11(3):162-170. doi: 10.47429/lmo.2021.11.3.162.
Reference
-
1. Lipowsky HH. Skalak R, Chien S, editors. Mechanics of blood flow in the microcirculation. Handbook of Bioengineering. 1987. New York: McGraw-Hill;18–25.2. Nichols WW, O'Rouke MF. McDonald's Blood Flow in Arteries Theoretical, Experimental and Clinical Principles. 1988. 4th ed. London: Arnold.3. Dinnar U. Cardiovascular Fluid Dynamics. 1981. Boca Raton: CRC Press.4. Yang WJ. Biothermal-Fluid Sciences Principles and Applications. 1989. New York: Hemisphere Pub. Corp.5. Munson BR, Young DF, Okiishi TH, Huebsch WW. Fundamentals of Fluid Mechanics. 2009. 6th ed. New York: John Wiley & Sons.6. Pries AR, Secomb TW, Gaehtgens P. Structure and hemodynamics of microvascular networks: heterogeneity and correlations. Am J Physiol. 1995. 269:H1713–H1722.7. Lipowsky HH. Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Blood rheology apects of the microcirculation. Handbook of Hemorheology and Hemodynamics. 2007. Washington, DC: IOS Press;307–321.8. Cocklet GR, Meiselman HJ. Baskurt OK, Hardeman MR, Rampling MW, Meiselman HJ, editors. Blood rheology. Handbook of Hemor-heology and Hemodynamics. 2007. Washington, DC: IOS Press;45–71.9. Fung YC. Biomechanics. 1981. New York: Spriner-Verlag.10. Stoltz JF, Singh M, Riha P. Hemorheology in Practice. 1999. Washington, DC: IOS Press.11. Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003. 29:435–450.12. Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999. 282:2035–2042.13. de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990. 81:107–117.14. Ditzel J, Kampmann J. Whole-blood viscosity, hematocrit and plasma protein in normal subjects at different ages. Acta Physiol Scand. 1971. 81:264–268.15. Rosenson RS, McCormick A, Uretz EF. Distribution of blood viscosity values and biochemical correlates in healthy adults. Clin Chem. 1996. 42:1189–1195.16. Mayer GA. Blood viscosity in healthy subjects and patients with coronary heart disease. Can Med Assoc J. 1964. 91:951–954.17. Letcher RL, Chien S, Pickering TG, Sealey JE, Laragh JH. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects: role of fibrinogen and concentration. Am J Med. 1981. 70:1195–1202.18. Litwin MS, Chapman K, Stoliar JB. Blood viscosity in the normal man. Surgery. 1970. 67:342–345.19. Rand PW, Lacombe E, Hunt HE, Austin WH. Viscosity of normal human blood under normothermic and hypothermic conditions. J Appl Physiol. 1964. 19:117–122.20. Chien S. Blood rheology in myocardial infarction and hypertension. Biorheology. 1986. 23:633–653.21. Lee BK, Durairaj A, Mehra A, Wenby RB, Meiselman HJ, Alexy T. Microcirculatory dysfunction in cardiac syndrome X: role of abnormal blood rheology. Microcirculation. 2008. 15:451–459.22. Tsuda Y, Satoh K, Kitadai M, Takahashi T. Hemorheologic profiles of plasma fibrinogen and blood viscosity from silent to acute and chronic cerebral infarctions. J Neurol Sci. 1997. 147:49–54.23. Coull BM, Beamer N, de Garmo P, et al. Chronic blood hyperviscosity in subjects with acute stroke, transient ischemic attack, and risk factors for stroke. Stroke. 1991. 22:162–168.24. Dormandy JA, Hoare E, Postlethwaite J. Importance of blood viscosity: rheological claudication. Proc R Soc Med. 1974. 67:446–447.25. Sloop GD, Mercante DE. Opposite effects of low-density and high-den-sity lipoprotein on blood viscosity in fasting subjects. Clin Hemorheol Microcirc. 1998. 19:197–203.26. Fantl P, Ward HA. Molecular weight of human fibrinogen derived from phosphorus determinations. Biochem J. 1965. 96:886–889.27. Pulanić D, Rudan I. The past decade: fibrinogen. Coll Antropol. 2005. 29:341–349.28. Moriarty PM, Gibson CA. Association between hematological parameters and high-density lipoprotein cholesterol. Curr Opin Cardiol. 2005. 20:318–323.29. Banerjee AK, Pearson J, Gilliland EL, et al. A six year prospective study of fibrinogen and other risk factors associated with mortality in stable claudicants. Thromb Haemost. 1992. 68:261–263.30. Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci (Lond). 1997. 92:473–479.31. Bachorik PS, Levy RI, Rifkind BM. Henry JB, editor. Lipids and dyslipoproteinemia. Clinical Diagnosis and Management by Laboratory Methods. 2001. 20th ed. Philadelphia: WB Saunders;1–2.32. Hall JE. Guyton and Hall Textbook of Medical Physiology. 2011. 12th ed. Philadlephia: WB Saunders.33. Cho YI, Kensey KR. Effects of the non-Newtonian viscosity of blood on flows in a diseased arterial vessel: part 1: steady flows. Biorheology. 1991. 28:241–262.34. Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998. 339:584–590.35. Drüeke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006. 355:2071–2084.36. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006. 355:2085–2098.37. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009. 361:2019–2032.38. Kameneva MV, Watach MJ, Borovetz HS. Gender difference in oxygen delivery index: potential link to development of cardiovascular diseases. Appl Cardiopulm Pathophysiol. 2000. 9:382–387.39. Usami S, Chien S, Gregersen MI. Viscometric characteristics of blood of the elephant, man, dog, sheep, and goat. Am J Physiol. 1969. 217:884–890.40. Alexy T, Pais E, Armstrong JK, Meiselman HJ, Johnson CS, Fisher TC. Rheologic behavior of sickle and normal red blood cell mixtures in sickle plasma: implications for transfusion therapy. Transfusion. 2006. 46:912–918.41. Kenyeres P, Juricskay I, Tarsoly P, et al. Low hematocrit per blood viscosity ratio as a mortality risk factor in coronary heart disease. Clin Hemorheol Microcirc. 2008. 38:51–56.42. Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009. 73:1983–1992.43. Malek AM, Izumo S, Alper SL. Modulation by pathophysiological stimuli of the shear stress-induced up-regulation of endothelial nitric oxide synthase expression in endothelial cells. Neurosurgery. 1999. 45:334–344. discussion 344-5.44. White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007. 362:1459–1467.45. Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis: insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988. 112:1018–1031.46. Malek AM, Jiang L, Lee I, Sessa WC, Izumo S, Alper SL. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun. 1999. 254:231–242.47. Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006. 103:15463–15468.48. Eng E, Ballermann BJ. Diminished NF-kappaB activation and PDGF-B expression in glomerular endothelial cells subjected to chronic shear stress. Microvasc Res. 2003. 65:137–144.49. Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005. 38:1949–1971.50. Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005. 12:5–15.51. Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998. 9:1018–1022.52. Merrill EW, Cokelet GC, Britten A, Wells RE Jr. Non-Newtonian rheology of human blood: effect of fibrinogen deduced by "subtraction". Circ Res. 1963. 13:48–55.53. Copley AL, King RG. Rheogoniometric viscosity measurements of whole human blood at minimal shear rates down to 0.0009 sec-1. Experientia. 1970. 26:904–905.54. Merrill EW, Gilliland ER, Cokelet G, Shin H, Britten A, Wells RE Jr. Rheology of human blood, near and at zero flow: effects of temperature and hematocrit level. Biophys J. 1963. 3:199–213.55. Merrill EW, Cheng CS, Pelletier GA. Yield stress of normal human blood as a function of endogenous fibrinogen. J Appl Physiol. 1969. 26:1–3.56. Yeow YL, Wickramasinghe SR, Leong YK, Han B. Model-independent relationships between hematocrit, blood viscosity, and yield stress derived from Couette viscometry data. Biotechnol Prog. 2002. 18:1068–1075.57. Picart C, Piau JM, Galliard H, Carpentier PH. Threshold of shear stress in human blood for healthy and sick subjects. J Mal Vasc. 1998. 23:113–118.58. Errill EW. Rheology of blood. Physiol Rev. 1969. 49:863–888.59. Zydney AL, Oliver JD III, Colton CK. A constitutive equation for the viscosity of stored red cell suspensions: effects of hematocrit, shear rate, and suspending phase. J Rheol. 1991. 35:1639–1680.60. Picart C, Piau JM, Galliard H, Carpentier PH. Blood yield stress and its Hematocrit Dependence. J Rheol. 1998. 42:1–12.61. Chien S, Usami S, Taylor HM, Lundberg JL, Gregersen MI. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol. 1966. 21:81–87.62. Morris CL, Smith CM 2nd, Blackshear PL Jr. A new method for measuring the yield stress in thin layers of sedimenting blood. Biophys J. 1987. 52:229–240.63. Picart C, Carpentier PH, Galliard H, Piau JM. Blood yield stress in systemic sclerosis. Am J Physiol. 1999. 276:H771–H777.64. Yu PK, Balaratnasingam C, Cringle SJ, McAllister IL, Provis J, Yu DY. Microstructure and network organization of the microvasculature in the human macula. Invest Ophthalmol Vis Sci. 2010. 51:6735–6743.65. Jung F, Pindur G, Hiebl B, Franke RP. Influence of capillary geometry on hypoperfusion-induced ischemia: a numerical study. Appl Cardiopulm Pathophysiol. 2010. 14:229–235.66. Benedict KF, Coffin GS, Barrett EJ, Skalak TC. Hemodynamic systems analysis of capillary network remodeling during the progression of type 2 diabetes. Microcirculation. 2011. 18:63–73.67. Shea SM, Raskova J. Glomerular hemodynamics and vascular structure in uremia: a network analysis of glomerular path lengths and maximal blood transit times computed for a microvascular model reconstructed from subserial ultrathin sections. Microvasc Res. 1984. 28:37–50.68. McManus BM, Allard MF, Yanagawa R. Rubin R, Strayer DS, Rubin E, editors. Hemodynamic Disorders. Rubin's Pathology: Clinicopathologic Foundations of Medicine. 2008. 5th ed. Baltimore: Lippincott Williams & Wilkins;229–230.69. Payman R, Lyon MJ. Rat utricular macula: blood flow and stereological assessment of capillary morphology. Ann Otol Rhinol Laryngol. 1993. 102:893–899.70. Arend O, Wolf S, Jung F, et al. Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991. 75:514–518.71. Lübbers DW. Microcirculation and O2 exchange through the skin surface: a theoretical analysis. Adv Exp Med Biol. 1994. 361:51–58.72. Lübbers DW, Baumgärtl H. Heterogeneities and profiles of oxygen pressure in brain and kidney as examples of the pO2 distribution in the living tissue. Kidney Int. 1997. 51:372–380.73. Chaturani P, Narasimman S. Theory for flow of Casson and Herschel-Bulkley fluids in cone-plate viscometers. Biorheology. 1988. 25:199–207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microvascular Lesions of the Vocal Folds in the Patients with Hoarseness
- Pushing Boundaries: Cardiac Computed Tomography Reveals Myocardial Microvascular Dysfunction in Patients With Diabetes
- Frequencies and Risk Factors for Microvascular Complications in Patients with Type 1 Diabetes Mellitus
- Osteocutaneous Free Flap Transfer by Microsurgical Technique
- Contribution of Microbleeds on Microvascular Magnetic Resonance Imaging Signal