Korean J Gastroenterol.
2013 Nov;62(5):278-287. 10.4166/kjg.2013.62.5.278.
Efficacy of Fenoverine and Trimebutine in the Management of Irritable Bowel Syndrome: Multicenter Randomized Double-blind Non-inferiority Clinical Study
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. ytjeen@korea.ac.kr
- 2Department of Internal Medicine, Gachon University School of Medicine, Incheon, Korea.
- 3Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea.
- 4Department of Internal Medicine, Inje University College of Medicine, Busan, Korea.
- 5Department of Internal Medicine, Kangwon National University College of Medicine, Chuncheon, Korea.
- 6Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 7Department of Internal Medicine, Wonkwang University College of Medicine, Iksan, Korea.
- KMID: 1775735
- DOI: http://doi.org/10.4166/kjg.2013.62.5.278
Abstract
- BACKGROUND/AIMS
Antispasmodic agents have been used in the management of irritable bowel syndrome. However, systematic reviews have come to different conclusions about the efficacy in irritable bowel syndrome. Fenoverine acts as a synchronizer of smooth muscle in modulating the intracellular influx of calcium. We compared fenoverine with trimebutine for the treatment of patients with IBS.
METHODS
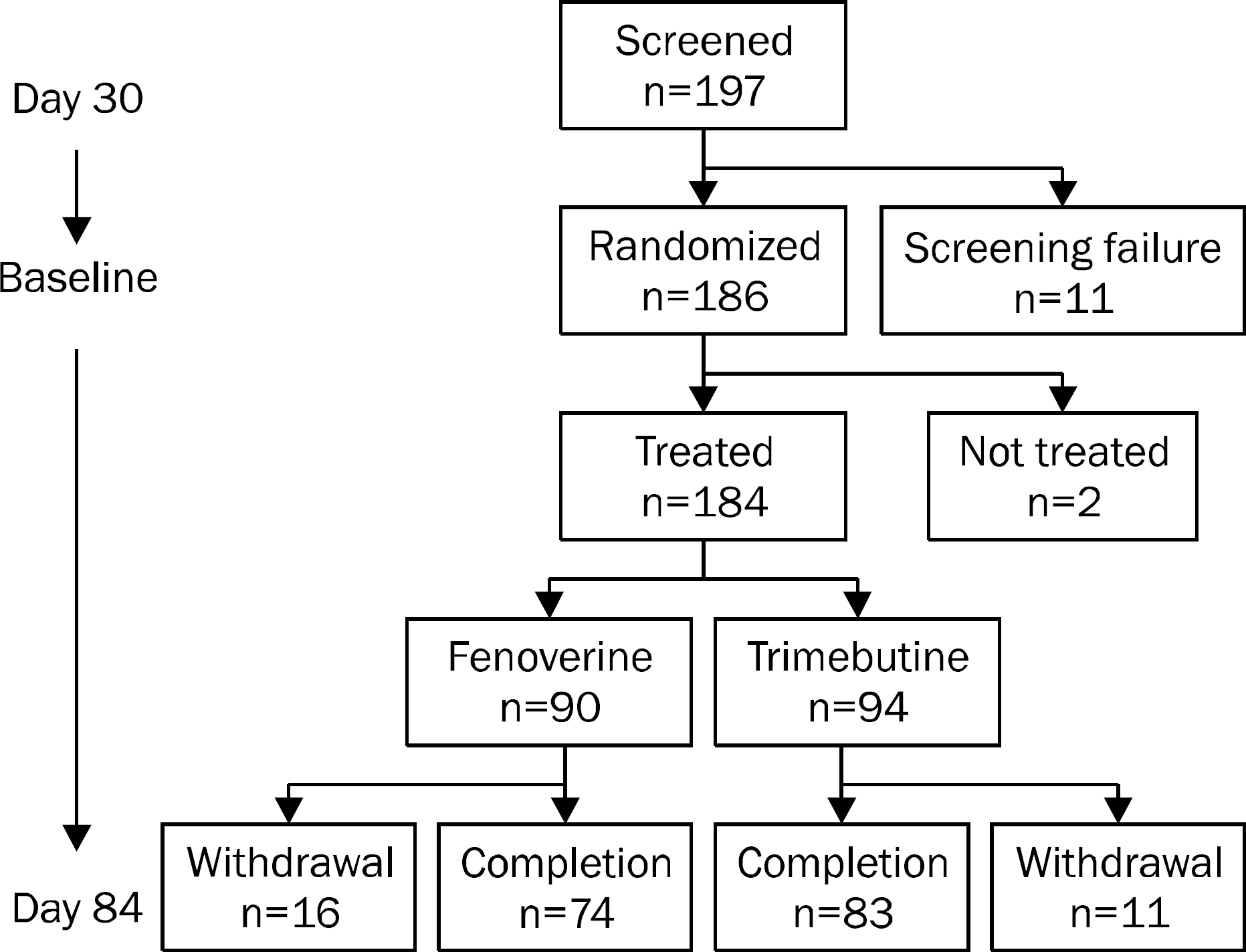
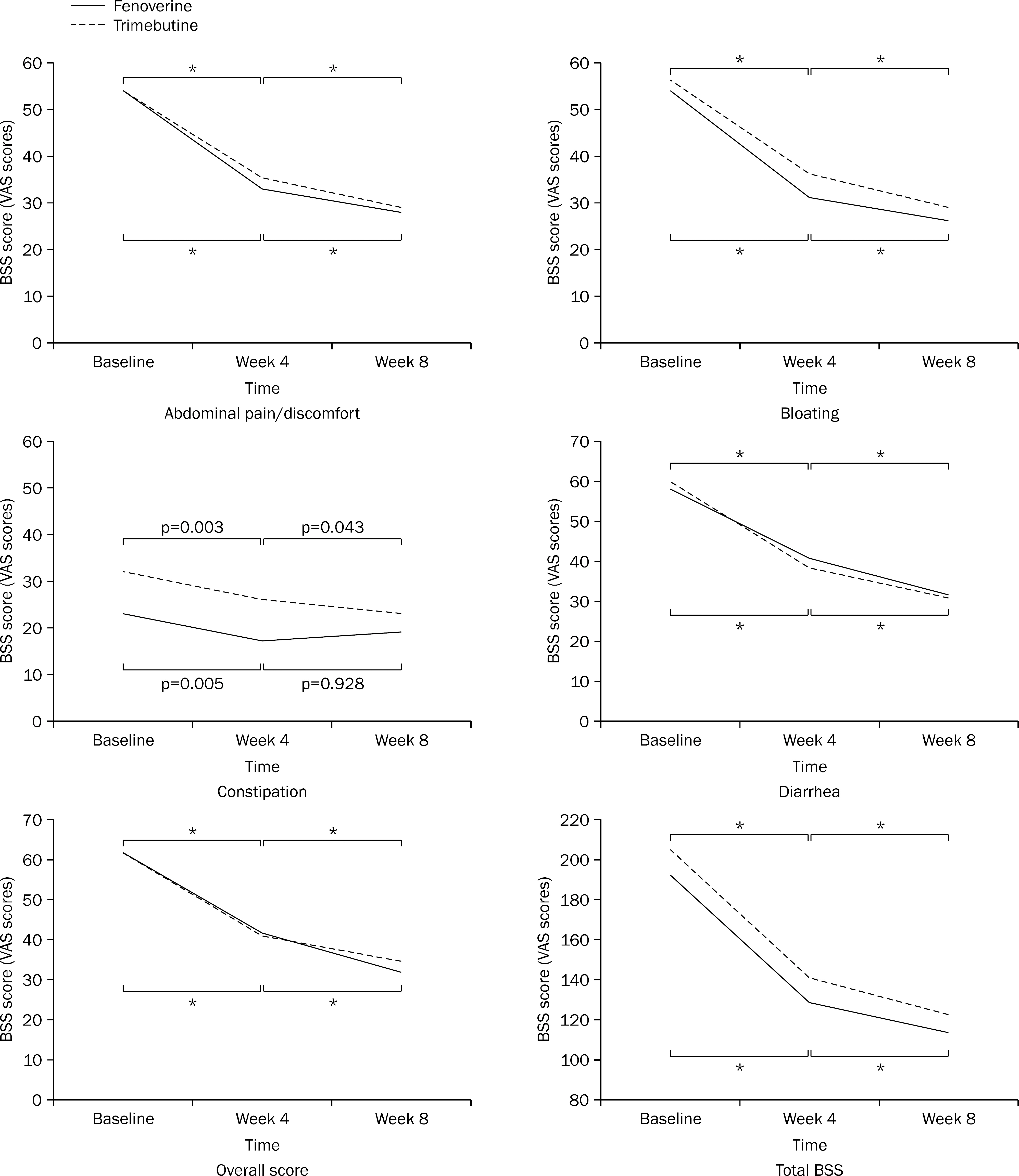
A multicenter, randomized, double-blind, non-inferiority clinical study was conducted to compared fenoverine with trimebutine. Subjects were randomized to receive either fenoverine (100 mg three times a day) or trimebutine (150 mg three times a day) for 8 weeks. A total of 197 patients were analyzed by the intention-to-treat approach. The primary endpoint was the proportion of patients who had 30% reduction in abdominal pain or discomfort measured by bowel symptom scale (BSS) score at week 8 compared to the baseline. The secondary endpoints were changes of abdominal bloating, diarrhea, constipation, overall and total scores of BSS, and overall satisfaction.
RESULTS
At week 8, fenoverine was shown to be non-inferior to trimebutine (treatment difference, 1.76%; 90% CI, -10.30-13.82; p=0.81); 69.23% (54 of 78 patients) of patients taking fenoverine and 67.47% (56 of 83 patients) of patients taking trimebutine showed 30% reduction in abdominal pain or discomfort compared to the baseline. There results of the secondary endpoints were also comparable between the fenoverine group and the trimebutine group.
CONCLUSIONS
Fenoverine is non-inferior to trimebutine for treating IBS in terms of both efficacy and tolerability.
MeSH Terms
-
Abdominal Pain/etiology
Adult
Constipation/etiology
Diarrhea/etiology
Double-Blind Method
Drug Administration Schedule
Female
Humans
Irritable Bowel Syndrome/complications/*drug therapy
Male
Middle Aged
Parasympatholytics/*therapeutic use
Phenothiazines/*therapeutic use
Severity of Illness Index
Treatment Outcome
Trimebutine/*therapeutic use
Parasympatholytics
Phenothiazines
Trimebutine
Figure
Cited by 1 articles
-
Effect of antispasmodic agents for the treatment of irritable bowel syndrome
Sang Heon Lee, Sam Ryong Jee
J Korean Med Assoc. 2018;61(7):428-434. doi: 10.5124/jkma.2018.61.7.428.
Reference
-
References
1. Choung RS, Locke GR 3rd. Epidemiology of IBS. Gastroenterol Clin North Am. 2011; 40:1–10.
Article2. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491.
Article3. Choung RS, Locke GR 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009; 104:1772–1779.
Article4. Sandler RS. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology. 1990; 99:409–415.
Article5. Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ 3rd. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991; 101:927–934.
Article6. Han SH, Lee OY, Bae SC, et al. Prevalence of irritable bowel syndrome in Korea: population-based survey using the Rome II criteria. J Gastroenterol Hepatol. 2006; 21:1687–1692.
Article7. Lee SY, Lee KJ, Kim SJ, Cho SW. Prevalence and risk factors for overlaps between gastroesophageal reflux disease, dyspepsia, and irritable bowel syndrome: a population-based study. Digestion. 2009; 79:196–201.
Article8. Park DW, Lee OY, Shim SG, et al. The differences in prevalence and sociodemographic characteristics of irritable bowel syndrome according to Rome II and Rome III. J Neurogastroenterol Motil. 2010; 16:186–193.
Article9. El-Serag HB, Olden K, Bjorkman D. Health-related quality of life among persons with irritable bowel syndrome: a systematic review. Aliment Pharmacol Ther. 2002; 16:1171–1185.
Article10. Mönnikes H. Quality of life in patients with irritable bowel syndrome. J Clin Gastroenterol. 2011; 45(Suppl):S98–S101.
Article11. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012; 303:G775–G785.
Article12. Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011; (8):CD003460.
Article13. Assisi RBP, Boggiano CA, Camarri E, Corsini G, Luminari M. An Italian prospective, double-blind, multicentre comparative assessment of fenoverine vs. tremebutine for the management of the irritable bowel syndrome. Internat J Clin Pract. 1989; 43:81–88.14. Camarri E. Fenoverine: smooth muscle synchronizer for the management of gastrointestinal conditions. II. A trimebutine- controlled, double-blind, crossover clinical evaluation. Curr Med Res Opin. 1986; 10:52–57.15. Mironneau J, Arnaudeau S, Mironneau C. Fenoverine inhibition of calcium channel currents in single smooth muscle cells from rat portal vein and myometrium. Br J Pharmacol. 1991; 104:65–70.
Article16. Choi MG. Management of irritable bowel syndrome. Korean J Gastroenterol. 2006; 47:125–130.17. Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001; 15:355–361.
Article18. Clavé P, Acalovschi M, Triantafillidis JK, et al. OBIS Study Investigators. Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2011; 34:432–442.
Article19. Wittmann T, Paradowski L, Ducrotté P, Bueno L, Andro Delestrain MC. Clinical trial: the efficacy of alverine citrate/simeticone combination on abdominal pain/discomfort in irritable bowel syndrome–a randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2010; 31:615–624.20. De Santis D, Marrazzo R, Losasso C, et al. Pharmacodynamic profile of fenoverine, a novel modulator of smooth muscle motility. Drugs Exp Clin Res. 1989; 15:37–42.21. Kim YT, Jung HC. Double-blind prospective controled study of fenoverine in irritable bowel syndrome. Korean J Gastroenterol. 1992; 24:263–267.22. Lee ST, Kim DG, Ahn DS. Assessment of fenoverine vs. mebeverine for the management of irritable bowel syndrome. Chonbuk Univ Med J. 1992; 16:75–81.23. Food and Drug Administration. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment; availability. Fed Regist. 2012; 77:32124–32125.24. Corsetti M, Tack J. FDA and EMA end points: which outcome end points should we use in clinical trials in patients with irritable bowel syndrome? Neurogastroenterol Motil. 2013; 25:453–457.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An open clinical trial on the effectiveness of trimebutine maleate (polybutine) in the irritable bowel syndrome: a comparative study of dosing twice and three times a day
- A clinical assessment on the effectiveness of trimebutine meleate(polybutine) in the irritable bowel syndrome
- Effect of antispasmodic agents for the treatment of irritable bowel syndrome
- Double blind clinical trial of tiropramide in irritable bowel syndrome
- The Incidence of Irritable Bowel Syndrome in Children Using the Rome III Criteria and the Effect of Trimebutine Treatment