Korean Circ J.
2013 Feb;43(2):73-79. 10.4070/kcj.2013.43.2.73.
Arterial Ageing
- Affiliations
-
- 1Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. SHPARK0530@yuhs.ac
- KMID: 1769640
- DOI: http://doi.org/10.4070/kcj.2013.43.2.73
Abstract
- Arterial ageing is characterized by age associated degeneration and sclerosis of the media layer of the large arteries. However, besides ageing, clinical conditions, which enhance oxidative stress and inflammation act to accelerate the degree of arterial ageing. In this review, we summarized the pathophysiology and contributing factors that accelerate arterial ageing. Among them, we focused on hypertension, the renin-angiotensin-aldosterone system and vascular inflammation which are modifiable causes of the arterial ageing process. Also, novel treatment targets derived from the disease models such as the Hutchinson Gilford Progeria Syndrome were reviewed.
MeSH Terms
Figure
Reference
-
1. Damjanov I, Linder J. Anderson's Pathology. 1996. 10th ed. St. Louis, MO: Mosby.2. Kumar V, Abbas A, Fausto N, Robbins SL, Cotran RS. Robbins and Cotran Pathologic Basis of Disease. 2005. 7th ed. Philadelphia, PA: Elsevier Saunders.3. Fishbein GA, Fishbein MC. Arteriosclerosis: rethinking the current classification. Arch Pathol Lab Med. 2009. 133:1309–1316.4. Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010. 10:Suppl 1. S213–S220.5. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001. 104:503–516.6. Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999. 33:116–123.7. Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991. 88:904–910.8. Huynh J, Nishimura N, Rana K, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011. 3:112ra122.9. Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann N Y Acad Sci. 2007. 1100:353–360.10. Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993. 88:77–81.11. Tsujimoto G, Lee CH, Hoffman BB. Age-related decrease in beta adrenergic receptor-mediated vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1986. 239:411–415.12. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010. 121:505–511.13. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001. 37:1236–1241.14. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007. 28:1462–1536.15. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007. 25:1105–1187.16. Benetos A, Adamopoulos C, Bureau JM, et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation. 2002. 105:1202–1207.17. Masugata H, Senda S, Murao K, et al. Association between urinary 8-hydroxydeoxyguanosine, an indicator of oxidative stress, and the cardio-ankle vascular index in hypertensive patients. J Atheroscler Thromb. 2012. 19:747–755.18. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012. 53:258–261.19. van Bussel BC, Schouten F, Henry RM, et al. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011. 58:588–595.20. Mahmud A, Feely J. Arterial stiffness and the renin-angiotensin-aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004. 5:102–108.21. Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001. 103:987–992.22. Takami T, Shigemasa M. Efficacy of various antihypertensive agents as evaluated by indices of vascular stiffness in elderly hypertensive patients. Hypertens Res. 2003. 26:609–614.23. Munakata M, Nagasaki A, Nunokawa T, et al. Effects of valsartan and nifedipine coat-core on systemic arterial stiffness in hypertensive patients. Am J Hypertens. 2004. 17(11 Pt 1):1050–1055.24. Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol. 2010. 160:1273–1292.25. Shahin Y, Khan JA, Chetter I. Angiotensin converting enzyme inhibitors effect on arterial stiffness and wave reflections: a meta-analysis and meta-regression of randomised controlled trials. Atherosclerosis. 2012. 221:18–33.26. Duprez DA. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation: a clinical review. J Hypertens. 2006. 24:983–991.27. Patarroyo Aponte MM, Francis GS. Effect of Angiotensin-converting enzyme inhibitors and Angiotensin receptor antagonists in atherosclerosis prevention. Curr Cardiol Rep. 2012. 14:433–442.28. Park S, Kim JB, Shim CY, et al. The influence of serum aldosterone and the aldosterone-renin ratio on pulse wave velocity in hypertensive patients. J Hypertens. 2007. 25:1279–1283.29. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009. 54:505–512.30. Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation. 2002. 106:2848–2853.31. Nehme JA, Lacolley P, Labat C, et al. Spironolactone improves carotid artery fibrosis and distensibility in rat post-ischaemic heart failure. J Mol Cell Cardiol. 2005. 39:511–519.32. Wang M, Zhang J, Jiang LQ, et al. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007. 50:219–227.33. Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002. 90:251–262.34. Ishibashi M, Hiasa K, Zhao Q, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004. 94:1203–1210.35. Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992. 148:2423–2428.36. Guo F, Liu J, Wang C, Liu N, Lu P. Fibrinogen, fibrin, and FDP induce C-reactive protein generation in rat vascular smooth muscle cells: pro-inflammatory effect on atherosclerosis. Biochem Biophys Res Commun. 2009. 390:942–946.37. Smith EB. Fibrinogen, fibrin and the arterial wall. Eur Heart J. 1995. 16:Suppl A. 11–14. discussion 14-5.38. Inoue N. Vascular C-reactive protein in the pathogenesis of coronary artery disease: role of vascular inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets. 2006. 6:227–231.39. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004. 176:111–116.40. Rodríguez-Mañas L, El-Assar M, Vallejo S, et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009. 8:226–238.41. Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001. 103:2531–2534.42. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005. 46:1118–1122.43. Nagano M, Nakamura M, Sato K, Tanaka F, Segawa T, Hiramori K. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005. 180:189–195.44. Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001. 38:399–403.45. Provan SA, Angel K, Semb AG, et al. Early prediction of increased arterial stiffness in patients with chronic inflammation: a 15-year followup study of 108 patients with rheumatoid arthritis. J Rheumatol. 2011. 38:606–612.46. Mäki-Petäjä KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006. 114:1185–1192.47. Yildiz M, Yildiz BS, Soy M, Tutkan H. Impairment of arterial distensibility in premenopausal women with systemic lupus erythematosus. Kardiol Pol. 2008. 66:1194–1199. discussion 1200-1.48. Galarraga B, Khan F, Kumar P, Pullar T, Belch JJ. Etanercept improves inflammation-associated arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford). 2009. 48:1418–1423.49. Kovacic JC, Moreno P, Hachinski V, Nabel EG, Fuster V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation. 2011. 123:1650–1660.50. Reunert J, Wentzell R, Walter M, et al. Neonatal progeria: increased ratio of progerin to lamin A leads to progeria of the newborn. Eur J Hum Genet. 2012. 20:933–937.51. Ragnauth CD, Warren DT, Liu Y, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010. 121:2200–2210.52. Lefèvre C, Auclair M, Boccara F, et al. Premature senescence of vascular cells is induced by HIV protease inhibitors: implication of prelamin A and reversion by statin. Arterioscler Thromb Vasc Biol. 2010. 30:2611–2620.53. Capell BC, Olive M, Erdos MR, et al. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci U S A. 2008. 105:15902–15907.54. Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet. 2005. 14:2959–2969.55. Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2005. 102:14416–14421.56. Mans RA, McMahon LL, Li L. Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience. 2012. 202:1–9.57. Hongo M, Kumazaki S, Izawa A, et al. Low-dose rosuvastatin improves arterial stiffness in high-risk Japanese patients with dyslipdemia in a primary prevention group. Circ J. 2011. 75:2660–2667.58. Cenni V, Capanni C, Columbaro M, et al. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur J Histochem. 2011. 55:e36.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Psychosocial Aspects of Normal Ageing
- Concept and Model of Successful Aging
- Evaluation of Skin Furrows in the Ageing Process using an Image Analysis System
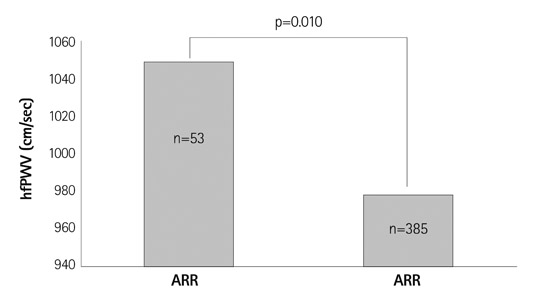
- Comparative Study of Elastic Fiber by Image Analysis System in Exposed and Nonexposed Human Skin
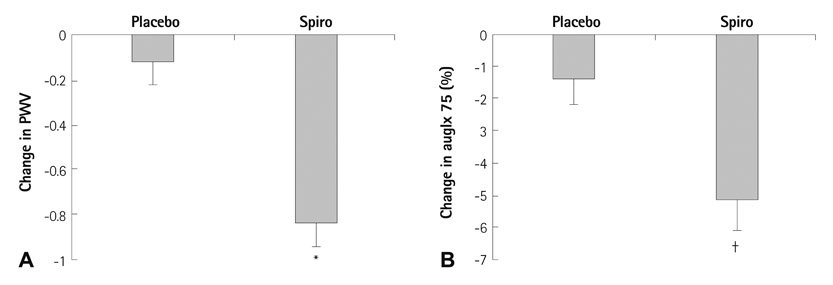
- Extracellular Vesicles and Immune System in Ageing and Immune Diseases