Korean J Physiol Pharmacol.
2012 Jun;16(3):159-165. 10.4196/kjpp.2012.16.3.159.
Pak1/LIMK1/Cofilin Pathway Contributes to Tumor Migration and Invasion in Human Non-Small Cell Lung Carcinomas and Cell Lines
- Affiliations
-
- 1Department of Anatomy, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea. anaroh@gnu.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- 3Department of Physiology, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- 4Department of Pathology, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- 5Department of Preventive Medicine, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju 660-290, Korea.
- 6Clinical Research Institute, Gyeongsang National University Hospital, Jinju 660-290, Korea.
- KMID: 1768006
- DOI: http://doi.org/10.4196/kjpp.2012.16.3.159
Abstract
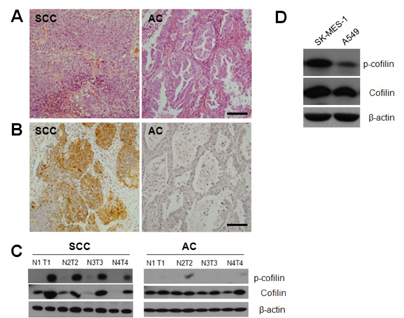
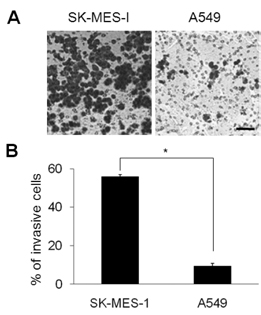
- Squamous cell carcinoma (SCC) and adenocarcinoma (AC) are the major histological types of non-small cell lung carcinoma (NSCLC). Although both SCCs and ACs have been characterized histologically and clinically, the precise mechanisms underlying their migration and invasion are not yet known. Here, we address the involvement in NSCLC of the p21-associated kinase1 (Pak1)/LIM kinase1 (LIMK1)/cofilin pathway, which recently has been reported to play a critical role in tumor migration and invasion. The Pak1/LIMK1/cofilin pathway was evaluated in tumors from SCC (n=35) and AC (n=35) patients and in SCC- and AC-type cell lines by western blotting, immunohistochemistry, and in vitro migration and invasion assays. The levels of phosphorylated Pak1, LIMK1, and cofilin in lung tumor tissues from SCC patients were increased as compared to normal tissues. In addition, immunohistochemistry showed greater expression of phosphorylated cofilin in SCC tissues. Expression of phosphorylated Pak1 and LIMK1 proteins was also significantly higher in SCC-type cells than in AC-type cells. Moreover, migration and invasion assays revealed that a higher percentage of SCC type cells exhibited migration and invasion compared to AC type cells. Migration was also decreased in LIMK1 knockdown SK-MES-1 cells. These findings suggest that the activation of the Pak1/LIMK1/cofilin pathway could preferentially contribute to greater tumor migration and invasion in SCC, relative to that in AC.
Keyword
MeSH Terms
Figure
Reference
-
1. Poleri C, Morero JL, Nieva B, Vázquez MF, Rodríguez C, de Titto E, Rosenberg M. Risk of recurrence in patients with surgically resected stage I non-small cell lung carcinoma: histopathologic and immunohistochemical analysis. Chest. 2003. 123:1858–1867.2. Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003. 3:55–63.3. Stupack DG, Cho SY, Klemke RL. Molecular signaling mechanisms of cell migration and invasion. Immunol Res. 2000. 21:83–88.4. Ding Y, Milosavljevic T, Alahari SK. Nischarin inhibits LIM kinase to regulate cofilin phosphorylation and cell invasion. Mol Cell Biol. 2008. 28:3742–3756.5. Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003. 72:743–781.6. Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006. 6:459–471.7. Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999. 1:253–259.8. Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil. 1998. 19:839–854.9. Vadlamudi RK, Kumar R. P21-activated kinases in human cancer. Cancer Metastasis Rev. 2003. 22:385–393.10. Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem Sci. 1999. 24:350–355.11. Yoshioka K, Foletta V, Bernard O, Itoh K. A role for LIM kinase in cancer invasion. Proc Natl Acad Sci USA. 2003. 100:7247–7252.12. Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999. 9:364–370.13. Zebda N, Bernard O, Bailly M, Welti S, Lawrence DS, Condeelis JS. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol. 2000. 151:1119–1128.14. Lee YJ, Mazzatti DJ, Yun Z, Keng PC. Inhibition of invasiveness of human lung cancer cell line H1299 by over-expression of cofilin. Cell Biol Int. 2005. 29:877–883.15. Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol. 1997. 7:162–167.16. Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009. 28:51–63.17. Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000. 275:36238–36244.18. Schraml P, Schwerdtfeger G, Burkhalter F, Raggi A, Schmidt D, Ruffalo T, King W, Wilber K, Mihatsch MJ, Moch H. Combined array comparative genomic hybridization and tissue microarray analysis suggest PAK1 at 11q13.5-q14 as a critical oncogene target in ovarian carcinoma. Am J Pathol. 2003. 163:985–992.19. Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004. 279:1422–1428.20. Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O’Brien T, Vucic D, Harris AL, Belvin M, Friedman LS, Blackwood EM, Koeppen H, Hoeflich KP. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci USA. 2011. 108:7177–7182.21. Adam L, Vadlamudi R, Mandal M, Chernoff J, Kumar R. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J Biol Chem. 2000. 275:12041–12050.22. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999. 285:895–898.23. Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000. 275:3577–3582.24. Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol. 2005. 171:349–359.25. Zebda N, Bernard O, Bailly M, Welti S, Lawrence DS, Condeelis JS. Phosphorylation of ADF/cofilin abolishes EGF-induced actin nucleation at the leading edge and subsequent lamellipod extension. J Cell Biol. 2000. 151:1119–1128.26. DesMarais V, Macaluso F, Condeelis J, Bailly M. Synergistic interaction between the Arp2/3 complex and cofilin drives stimulated lamellipod extension. J Cell Sci. 2004. 117:3499–3510.27. Sinha P, Hütter G, Köttgen E, Dietel M, Schadendorf D, Lage H. Increased expression of epidermal fatty acid binding protein, cofilin, and 14-3-3-sigma (stratifin) detected by two-dimensional gel electrophoresis, mass spectrometry and microsequencing of drug-resistant human adenocarcinoma of the pancreas. Electrophoresis. 1999. 20:2952–2960.28. Gunnersen JM, Spirkoska V, Smith PE, Danks RA, Tan SS. Growth and migration markers of rat C6 glioma cells identified by serial analysis of gene expression. Glia. 2000. 32:146–154.29. Castro MA, Dal-Pizzol F, Zdanov S, Soares M, Müller CB, Lopes FM, Zanotto-Filho A, da Cruz Fernandes M, Moreira JC, Shacter E, Klamt F. CFL1 expression levels as a prognostic and drug resistance marker in nonsmall cell lung cancer. Cancer. 2010. 116:3645–3655.30. Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer. 2007. 7:429–440.31. Sidani M, Wessels D, Mouneimne G, Ghosh M, Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, Eddy R, Soll D, Condeelis J. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol. 2007. 179:777–791.32. Sagawa M, Saito Y, Takahashi S, Usuda K, Kamma K, Sato M, Ota S, Nagamoto N, Fujimura S, Nakada T, Hashimoto K, Suda H, Imai T, Saito H. Clinical and prognostic assessment of patients with resected small peripheral lung cancer lesions. Cancer. 1990. 66:2653–2657.33. Bhattacharya R, Kowalski J, Larson AR, Brock M, Alani RM. Id1 promotes tumor cell migration in nonsmall cell lung cancers. J Oncol. 2010. 2010:856105.34. Cotran RS, Kumar V, Robbins S. Robbins pathologic basis of disease. 1989. 4th ed. Philadelphia: W.B. Sanders Company;797–804.35. Shah SA, Spinale FG, Ikonomidis JS, Stroud RE, Chang EI, Reed CE. Differential matrix metalloproteinase levels in adenocarcinoma and squamous cell carcinoma of the lung. J Thorac Cardiovasc Surg. 2010. 139:984–990.36. Pinto CA, Carvalho PE, Antonângelo L, Garippo A, Da Silva AG, Soares F, Younes R, Beyruti R, Takagaki T, Saldiva P, Vollmer RT, Capelozzi VL. Morphometric evaluation of tumor matrix metalloproteinase 9 predicts survival after surgical resection of adenocarcinoma of the lung. Clin Cancer Res. 2003. 9:3098–3104.37. Peters MG, Farías E, Colombo L, Filmus J, Puricelli L, Bal de Kier Joffé E. Inhibition of invasion and metastasis by glypican-3 in a syngeneic breast cancer model. Breast Cancer Res Treat. 2003. 80:221–232.38. Aviel-Ronen S, Lau SK, Pintilie M, Lau D, Liu N, Tsao MS, Jothy S. Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Mod Pathol. 2008. 21:817–825.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Anti-tumor Effect of Macrolides on the Extracellular Matrix Invasion and Cell Adhesion in Human Non-small Cell Lung Cancer Cells
- Expression of the FHIT gene Located in Chromosome 3p14.2 in Human Lung Cancer Cell Lines
- MicroRNA-373 Inhibits Cell Proliferation and Invasion via Targeting BRF2 in Human Non-small Cell Lung Cancer A549 Cell Line
- Integrin alphavbeta3, alpha5beta1 Effects on Cell Proliferation and Migration in Human Osteosarcoma
- Effect of BMI1 Knockdown on Cell Proliferation, Apoptosis, Invasiveness, and Migration of U251 Glioma Cells