Yonsei Med J.
2009 Aug;50(4):576-581. 10.3349/ymj.2009.50.4.576.
Malignant Glioma Arising at the Site of an Excised Cerebellar Hemangioblastoma after Irradiation in a von Hippel-Lindau Disease Patient
- Affiliations
-
- 1Department of Pathology, Dankook University College of Medicine, Cheonan, Korea. myongnh@dankook.ac.kr
- 2Department of Neurosurgery, Kyunghee University Hospital, Seoul, Korea.
- KMID: 1758620
- DOI: http://doi.org/10.3349/ymj.2009.50.4.576
Abstract
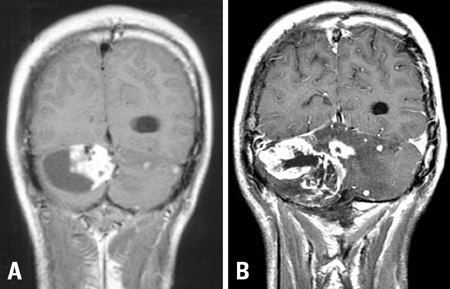
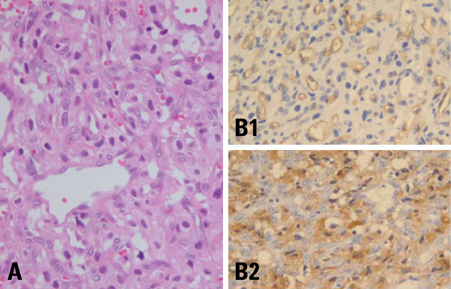
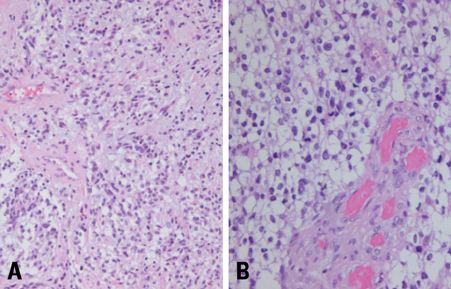
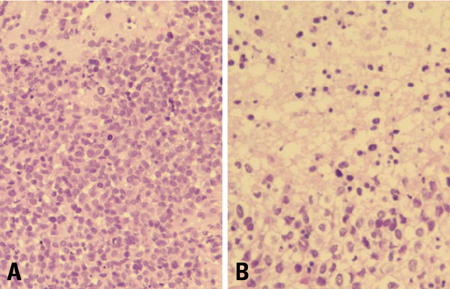
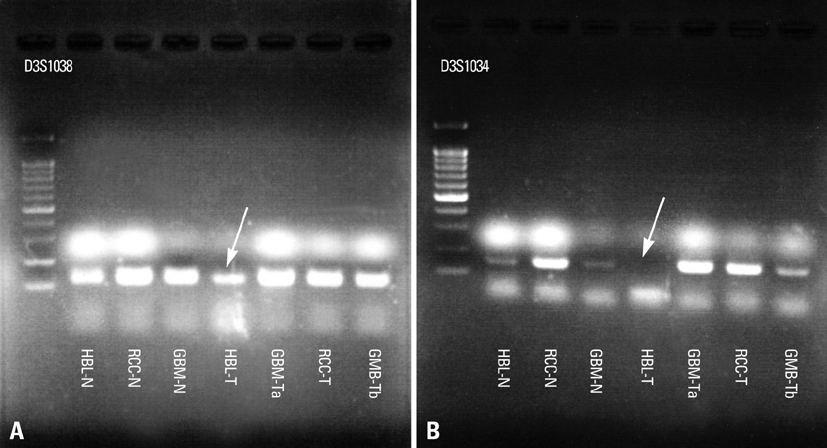
- We describe herein a malignant glioma arising at the site of the resected hemangioblastoma after irradiation in a patient with von Hippel-Lindau disease (VHL). The patient was a 25 year-old male with multiple hemangioblastomas at the cerebellum and spinal cord, multiple pancreatic cysts and a renal cell carcinoma; he was diagnosed as having VHL disease. The largest hemangioblastoma at the right cerebellar hemisphere was completely removed, and he received high-dose irradiation postoperatively. The tumor recurred at the same site 7 years later, which was a malignant glioma with no evidence of hemangioblastoma. The malignant glioma showed molecular genetic profiles of radiation-induced tumors because of its diffuse p53 immunostaining and the loss of p16 immunoreactivity. The genetic study to find the loss of heterozygosity (LOH) of VHL gene revealed that only the cerebellar hemangioblastoma showed allelic losses for the gene. To the best of our knowledge, this report is the first to show a malignant glioma that developed in a patient with VHL disease after radiation therapy at the site of an excised hemangioblastoma. This report also suggests that radiation therapy should be performed very carefully in VHL patients with hemangioblastomas.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Trends of Presentation and Clinical Outcome of Treated Renal Angiomyolipoma
Kyo Chul Koo, Won Tae Kim, Won Sik Ham, Jin Sun Lee, Hee Jeong Ju, Young Deuk Choi
Yonsei Med J. 2010;51(5):728-734. doi: 10.3349/ymj.2010.51.5.728.
Reference
-
1. Rubinstein JL, Yaari H. von Hippel-Lindau and the genetics of astrocytoma. J Natl Cancer Inst. 1994. 86:142–143.
Article2. Cornish D, Pont A, Minor D, Coombs JL, Bennington J. Metastatic islet cell tumor in von Hippel-Lindau disease. Am J Med. 1984. 77:147–150.
Article3. Ng HK, Tse JY, Poon WS. Cerebellar astrocytoma associated with von Hippel-Lindau disease: case report with molecular findings. Br J Neurosurg. 1999. 13:504–507.
Article4. Ertas G, Altundag MB, Ucer AR, Cankal F, Altundag K. Treatment of recurrent cerebellar hemangioblastoma with external radiotherapy in a patient with von Hippel-Lindau disease: a case report and review of the literature. J Neurooncol. 2005. 73:273–275.
Article5. Aksu G, Ulutin C, Fayda M, Saynak M. Cerebellar and multiple spinal hemangioblastomas and intraventricular meningioma managed with subtotal resection and external beam radiotherapy; report of a case with literature review. J BUON. 2005. 10:405–409.6. Brat DJ, James D, Jedlicka AE, Connolly DC, Chang E, Castellani RJ, et al. Molecular genetic alterations in radiation-induced astrocytomas. Am J Pathol. 1999. 154:1431–1438.
Article7. Kanno H, Shuin T, Kondo K, Yamamoto I, Ito S, Shinonaga M, et al. Somatic mutations of the von Hippel-Lindau tumor suppressor gene and loss of heterozygosity on chromosome 3p in human glial tumors. Cancer Res. 1997. 57:1035–1038.8. Yang SY, Wang KC, Cho BK, Kim YY, Lim SY, Park SH, et al. Radiation-induced cerebellar glioblastoma at the site of a treated medulloblastoma: case report. J Neurosurg. 2005. 102(4):Suppl. 417–422.
Article9. Cahan WG, Woodard HQ, Higinbotham NL, Stewart FW, Coley BL. Sarcoma arising in irradiated bone: report of eleven cases. 1948. Cancer. 1998. 82:8–34.10. Marus G, Levin CV, Rutherfoord GS. Malignant glioma following radiotherapy for unrelated primary tumors. Cancer. 1986. 58:886–894.
Article11. Evans DG, Birch JM, Ramsden RT, Sharif S, Baser ME. Malignant transformation and new primary tumours after therapeutic radiation for benign disease: substantial risks in certain tumour prone syndromes. J Med Genet. 2006. 43:289–294.12. Suzuki K, Ojima M, Kodama S, Watanabe M. Radiation-induced DNA damage and delayed induced genomic instability. Oncogene. 2003. 22:6988–6993.13. Morgan WF, Day JP, Kaplan MI, McGhee EM, Limoli CL. Genomic instability induced by ionizing radiation. Radiat Res. 1996. 146:247–258.
Article14. Kaschten B, Flandroy P, Reznik M, Hainaut H, Stevenaert A. Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg. 1995. 83:154–162.15. Simmons NE, Laws ER Jr. Glioma occurrence after sellar irradiation: case report and review. Neurosurgery. 1998. 42:172–178.16. Nakamizo A, Nishio S, Inamura T, Koga H, Yamabe K, Kuba H, et al. Evolution of malignant cerebellar astrocytoma at the site of a treated medulloblastoma: report of two cases. Acta Neurochir (Wien). 2001. 143:697–700.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-Term Follow-Up Clinical Courses of Cerebellar Hemangioblastoma in von Hippel-Lindau Disease : Two Case Reports and a Literature Review
- Three Cases of Cerebellar Hemangioblastoma in a von Hippel-Lindau Family: Case Report
- A Case of Von Hippel-Lindau Disease
- Von Hippel-Lindau Disease Manifestating as Recurrent Cerebellar Hemangioblastoma: A Case Report
- Multifocal Spinal Hemangioblastoma in von Hippel-Lindau Syndrome: A Case Report and Literature Review