Yonsei Med J.
2009 Aug;50(4):569-575. 10.3349/ymj.2009.50.4.569.
The Soluble Tumor Necrosis Factor-Alpha Receptor Suppresses Airway Inflammation in a Murine Model of Acute Asthma
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. cmcksc@catholic.ac.kr
- KMID: 1758619
- DOI: http://doi.org/10.3349/ymj.2009.50.4.569
Abstract
- PURPOSE
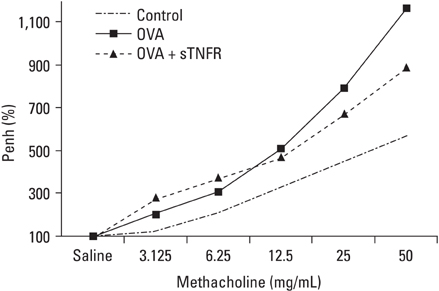
Tumor necrosis factor-alpha (TNF-alpha) is a proinflammatory cytokine that has been implicated in many aspects of the airway pathology in asthma. TNF-alpha blocking strategies are now being tried in asthma patients. This study investigated whether TNF-alpha blocking therapy inhibits airway inflammation and airway hyperresponsiveness (AHR) in a mouse model of asthma. We also evaluated the effect of TNF-alpha blocking therapy on cytokine production and adhesion molecule expression. MATERIALS AND METHODS: Ovalbumin (OVA) sensitized BALB/c female mice were exposed to intranasal OVA administration on days 31, 33, 35, and 37. Mice were treated intraperitoneally with soluble TNF-alpha receptor (sTNFR) during the OVA challenge. RESULTS: There were statistically significant decreases in the numbers of total cell and eosinophil in bronchoalveolar lavage fluid (BALF) in the sTNFR treated group compared with the OVA group. However, sTNFR-treatment did not significantly decrease AHR. Anti-inflammatory effect of sTNFR was accompanied with reduction of T helper 2 cytokine levels including interleukin (IL)-4, IL-5 and IL-13 in BALF and vascular cell adhesion molecule 1 expression in lung tissue. CONCLUSION: These results suggest that sTNFR treatment can suppress the airway inflammation via regulation of Th2 cytokine production and adhesion molecule expression in bronchial asthma.
MeSH Terms
-
Animals
Anti-Asthmatic Agents/*therapeutic use
Asthma/*drug therapy/*immunology
Blotting, Western
Bronchi/drug effects
Bronchial Hyperreactivity
Bronchoalveolar Lavage Fluid/immunology
Enzyme-Linked Immunosorbent Assay
Female
Immunohistochemistry
Inflammation/*drug therapy
Interleukin-13/metabolism
Interleukin-4/metabolism
Interleukin-5/metabolism
Mice
Mice, Inbred BALB C
Ovalbumin/pharmacology
Tumor Necrosis Factor-alpha/*therapeutic use
Figure
Cited by 1 articles
-
Association between Cystic Fibrosis Transmembrane Conductance Regulator Gene Mutations and Susceptibility for Childhood Asthma in Korea
Kyung Won Kim, Ji Hyun Lee, Min Goo Lee, Kyung Hwan Kim, Myung Hyun Sohn, Kyu-Earn Kim
Yonsei Med J. 2010;51(6):912-917. doi: 10.3349/ymj.2010.51.6.912.
Reference
-
1. Foster PS, Martinez-Moczygemba M, Huston DP, Corry DB. Interleukins-4, -5, and -13: emerging therapeutic targets in allergic disease. Pharmacol Ther. 2002. 94:253–264.
Article2. Meiler F, Zimmermann M, Blaser K, Akdis CA, Akdis M. T-cell subsets in the pathogenesis of human asthma. Curr Allergy Asthma Rep. 2006. 6:91–96.3. Lukacs NW, Strieter RM, Chensue SW, Widmer M, Kunkel SL. TNF-alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol. 1995. 154:5411–5417.4. Slungaard A, Vercellotti GM, Walker G, Nelson RD, Jacob HS. Tumor necrosis factor alpha/cachectin stimulates eosinophil oxidant production and toxicity towards human endothelium. J Exp Med. 1990. 171:2025–2041.
Article5. Scheurich P, Thoma B, Ucer U, Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987. 138:1786–1790.6. Lassalle P, Gosset P, Delneste Y, Tsicopoulos A, Capron A, Joseph M, et al. Modulation of adhesion molecule expression on endothelial cells during the late asthmatic reaction: role of macrophage-derived tumour necrosis factor-alpha. Clin Exp Immunol. 1993. 94:105–110.
Article7. Pennings HJ, Kramer K, Bast A, Buurman WA, Wouters EF. Tumour necrosis factor-alpha induces hyperreactivity in tracheal smooth muscle of the guinea-pig in vitro. Eur Respir J. 1998. 12:45–49.
Article8. Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax. 2005. 60:1012–1018.
Article9. Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007. 119:1337–1348.
Article10. Berry MA, Hargadon B, Shelley M, Parker D, Shaw DE, Green RH, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006. 354:697–708.
Article11. Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, et al. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med. 2006. 174:753–762.
Article12. Pennica D, Nedwin GE, Hayflick JS, Seeburg PH, Derynck R, Palladino MA, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984. 312:724–729.
Article13. Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med. 2000. 343:338–344.
Article14. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001. 344:907–916.
Article15. Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999. 340:1398–1405.
Article16. Bryan SA, Leckie MJ, Hansel TT, Barnes PJ. Novel therapy for asthma. Expert Opin Investig Drugs. 2000. 9:25–42.17. Holgate ST. Cytokine and anti-cytokine therapy for the treatment of asthma and allergic disease. Cytokine. 2004. 28:152–157.18. Amrani Y, Chen H, Panettieri RA Jr. Activation of tumor necrosis factor receptor 1 in airway smooth muscle: a potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir Res. 2000. 1:49–53.19. Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med. 1995. 152:76–80.20. Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, Chrousos GP, et al. Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor. J Clin Endocrinol Metab. 1999. 84:2834–2839.
Article21. Amrani Y, Panettieri RA Jr, Frossard N, Bronner C. Activation of the TNF alpha-p55 receptor induces myocyte proliferation and modulates agonist-evoked calcium transients in cultured human tracheal smooth muscle cells. Am J Respir Cell Mol Biol. 1996. 15:55–63.22. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993. 122:103–111.
Article23. Broide DH, Stachnick G, Castaneda D, Nayar J, Sriramarao P. Inhibition of eosinophilic inflammation in allergen-challenged TNF receptor p55/p75--and TNF receptor p55-deficient mice. Am J Respir Cell Mol Biol. 2001. 24:304–311.
Article24. Rudmann DG, Moore MW, Tepper JS, Aldrich MC, Pfeiffer JW, Hogenesch H, et al. Modulation of allergic inflammation in mice deficient in TNF receptors. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1047–L1057.
Article25. Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur Respir J. 2002. 19:879–885.
Article26. Chen FH, Samson KT, Miura K, Ueno K, Odajima Y, Shougo T, et al. Airway remodeling: a comparison between fatal and nonfatal asthma. J Asthma. 2004. 41:631–638.
Article27. Bates J, Irvin C, Brusasco V, Drazen J, Fredberg J, Loring S, et al. The use and misuse of Penh in animal models of lung disease. Am J Respir Cell Mol Biol. 2004. 31:373–374.
Article28. Glaab T, Ziegert M, Baelder R, Korolewitz R, Braun A, Hohlfeld JM, et al. Invasive versus noninvasive measurement of allergic and cholinergic airway responsiveness in mice. Respir Res. 2005. 6:139.
Article29. Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990. 346:274–276.
Article30. Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005. 102:6467–6472.
Article31. Tartaglia LA, Goeddel DV, Reynolds C, Figari IS, Weber RF, Fendly BM, et al. Stimulation of human T-cell proliferation by specific activation of the 75-kDa tumor necrosis factor receptor. J Immunol. 1993. 151:4637–4641.32. Baram D, Vaday GG, Salamon P, Drucker I, Hershkoviz R, Mekori YA. Human mast cells release metalloproteinase-9 on contact with activated T cells: juxtacrine regulation by TNF-alpha. J Immunol. 2001. 167:4008–4016.
Article33. Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007. 120:48–55.
Article34. Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993. 150:353–360.35. Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007. 4:269–275.36. Panettieri RA Jr, Lazaar AL, Puré E, Albelda SM. Activation of cAMP-dependent pathways in human airway smooth muscle cells inhibits TNF-alpha-induced ICAM-1 and VCAM-1 expression and T lymphocyte adhesion. J Immunol. 1995. 154:2358–2365.37. Kobayashi T, Hashimoto S, Imai K, Amemiya E, Yamaguchi M, Yachi A, et al. Elevation of serum soluble intercellular adhesion molecule-1 (sICAM-1) and sE-selectin levels in bronchial asthma. Clin Exp Immunol. 1994. 96:110–115.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Korean Red Ginseng Extracts on Airway Hyperresponsiveness and Inflammation in a Murine Asthma Model
- Serum Level of TNF-alpha and Soluble TNF Receptor I in Infants with RDS and Their Significance as a Prospective Indicator for Development of Infantile Asthma
- Circulating Levels of Interleukin-6 and Soluble Interleukin-6 Receptor in Acute Asthma
- Effects of Diet-Induced Mild Obesity on Airway Hyperreactivity and Lung Inflammation in Mice
- Effect of DHEA on airway hyperresponsiveness and inflammation in murine model of asthma