Cancer Res Treat.
2005 Feb;37(1):63-70.
Immunization with Adenoviral Vectors Carrying Recombinant IL-12 and E7 Enhanced the Antitumor Immunity against Human Papillomavirus 16-associated Tumor
- Affiliations
-
- 1Catholic Research Institutes of Medical Science, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 2Department of Obstetrics and Genecology, The Catholic University of Korea College of Medicine, Seoul, Korea. ahnws@catholic.ac.kr
- 3Department of Medicine, The Catholic University of Daegu, College of Medicine, Daegu, Korea.
- 4BMS Korea Research Center, Seoul, Korea.
Abstract
- PURPOSE
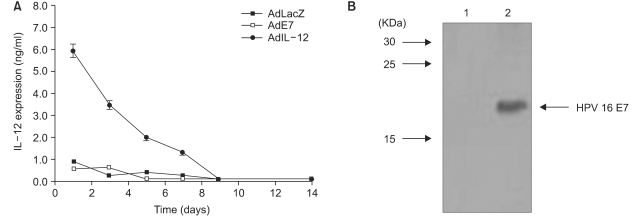
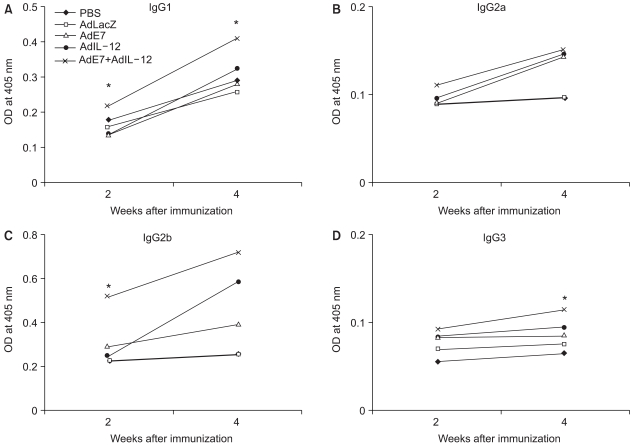
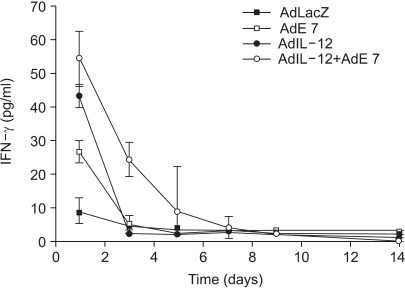
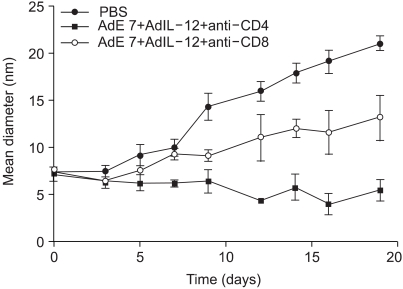
Human papillomavirus (HPV) infection has a significant role in cervical carcinogenesis, and HPV oncoprotein E7 plays an important part in the formation and maintenance of cervical cancer. Interleukin-12 (IL-12) has been reported to induce a cellular immune response, and to suppress the tumor growth and the E7 production. Here we describe the use of adenoviral delivery of the HPV 16 E7 subunit (AdE7) along with adenoviral delivery of IL-12 (AdIL-12) in mice with HPV-associated tumors. MATERIALS AND METHODS: Mice were injected with TC-1 cells to establish TC-1 tumor, and then they were immunized with AdIL-12 and/or AdE7 intratumorally. The anti tumor effects induced by AdIL-12 and/or E7 were evaluated by measuring the size of the tumor. E7-specific antibody and INF-gamma production in sera, and the T-helper cell proliferative responses were then measured. Cytotoxic T-lymphocyte (CTL) and T cell subset depletion studies were also performed. RESULTS: Combined AdIL-12 and AdE7 infection at the tumor sites significantly enhanced the antitumor effects more than that of AdIL-12 or AdE7 single infection. This combined infection resulted in regression of the 9 mm sized tumors in 80% of animals as compare to the PBS group. E7-specific antibody and INF-gamma production in the sera, and the T-helper cell proliferative responses were significantly higher with coinfection of AdIL-12 and AdE7 than with AdIL-12 or AdE7 alone. CTL response induced by AdIL-12 and AdE7 in the coinjected group suggested that tumor suppression was mediated by mostly CD8+ and only a little by the CD4+ T cells. CONCLUSION: IL-12 and E7 application using adenovirus vector showed antitumor immunity effects against TC-1 tumor, and this system could be use in clinical applications for HPV-associated cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991; 147:874–882. PMID: 1713608.2. Gubler U, Chua AO, Schoenhaut DS, Dwyer CM, McComas W, Motyka R, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991; 88:4143–4147. PMID: 1674604.
Article3. Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995; 146:423–431. PMID: 8839141.
Article4. Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000; 19:3530–3541. PMID: 10899108.
Article5. Vandenbroeck K, Alloza I, Gadina M, Matthys P. Inhibiting cytokines of the interleukin-12 family: recent advances and novel challenges. J Pharm Pharmacol. 2004; 56:145–160. PMID: 15005873.
Article6. Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, et al. Natural killer cell stimulatory factor (interleukin-12 (IL-12)) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4 producing Th cells. J Exp Med. 1993; 177:1199–1204. PMID: 8096238.7. Siders WM, Wright PW, Hixon JA, Alvord WG, Back TC, Wiltrout RH, et al. T cell and NK cell-independent inhibition of hepatic metastases by systemic administration of an IL-12-expressing recombinant adenovirus. J Immunol. 1998; 160:5465–5474. PMID: 9605149.8. Tahara H, Zeh HJ 3rd, Storkus WJ, Pappo I, Watkins SC, Gubler U, et al. Fibroblasts genetically engineered to secrete interleukin-12 can suppress tumor growth and induce antitumor growth and induce anti-tumor immunity to a murine melanoma in vivo. Cancer Res. 1994; 54:182–189. PMID: 7903204.9. Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000; 97:2208–2213. PMID: 10681459.
Article10. Puisieux I, Odin L, Poujol D, Moingeon P, Tartaglia J, Cox W, et al. Canarypox virus-mediated interleukin 12 gene transfer into murine mammary adenocarcinoma induces tumor suppression and long-term antitumoral immunity. Hum Gene Ther. 1998; 9:2481–2492. PMID: 9853515.
Article11. Paul D, Qazilbash MH, Song K, Xu H, Sinha BK, Liu J, et al. Construction of a recombinant adeno-associated virus (rAAV) vector expressing murine interleukin-12 (IL-12). Cancer Gene Ther. 2000; 7:308–315. PMID: 10770641.
Article12. Rakhmilevich AL, Janssen K, Turner J, Culp J, Yang NS. Cytokine gene therapy of cancer using gene gun technology: superior antitumor activity of interleukin-12. Hum Gene Ther. 1997; 8:1303–1311. PMID: 9295125.
Article13. Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu HK, Xiao X, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000; 74:2888–2894. PMID: 10684306.
Article14. Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996; 347:1523–1527. PMID: 8684105.
Article15. Boursnell ME, Rutherford E, Hickling JK, Rollinson EA, Munro AJ, Rolley N, et al. Construction and characterisation of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996; 14:1485–1494. PMID: 9014288.
Article16. Ahn WS, Bae SM, Kim TY, Kim TG, Lee JM, Namkoong SE, et al. A therapy modality using recombinant IL-12 adenovirus plus E7 protein in a human papillomavirus 16 E6/E7-associated cervical cancer animal model. Hum Gene Ther. 2003; 14:1389–1399. PMID: 14577920.
Article17. Kim TY, Myoung HJ, Kim JH, Moon IS, Kim TG, Ahn WS, et al. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8 T+ cells in protection. Cancer Res. 2002; 62:7234–7240. PMID: 12499264.18. Bae SM, Kim YW, Yoon JH, Yoo JY, Seo YS, Nam SL, et al. Cell-specific growth inhibition of human cervical cancer cell by recombinant adenovirus p53 in vitro and in vivo. Cancer Res Treat. 2003; 35:181–190.
Article19. Mitchell MF, Hamada K, Sastry KJ, Sarkar A, Tortolero-Luna G, Wharton JT, et al. Transgene expression in the rhesus cervix mediated by an adenovirus expressing beta-galactosidase. Am J Obstet Gynecol. 1996; 174:1094–1101. PMID: 8623835.20. Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996; 56:21–26. PMID: 8548765.21. Gambotto A, Tuting T, McVey DL, Kovesdi I, Tahara H, Lotze MT, et al. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther. 1999; 6:45–53. PMID: 10078963.
Article22. Fernando GJ, Murray B, Zhou J, Frazer IH. Expression, purification and immunological characterization of the transforming protein E7, from cervical cancer-associated human papillomavirus type 16. Clin Exp Immunol. 1999; 115:397–403. PMID: 10193409.
Article23. Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001; 61:3698–3703. PMID: 11325841.24. Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT. Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res. 2000; 60:5456–5463. PMID: 11034088.25. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997; 15:749–795. PMID: 9143706.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improved immunodetection of human papillomavirus E7
- Generation of Monoclonal Antibodies Against Human Papillomavirus Type16 E7 Protein : Usefulness for Various E7 Detection Systems
- Expression and localization of human papillomavirus type 16 E6 and E7 open reading frame proteins in human epidermal keratinocyte
- Expression of Human Papillomavirus Type 16, Prototype and Natural Variant E7 Proteins using Baculovirus Expression System
- Degradation of the Retinoblastoma Tumor Suppressor Protein by the Human Papillomavirus-16 E7 Variants