J Vet Sci.
2014 Mar;15(1):167-171. 10.4142/jvs.2014.15.1.167.
Relationship between pregnancy rate and serum progesterone concentration in cases of porcine embryo transfer
- Affiliations
-
- 1Department of Theriogenology and Biotechnology, College of Veterinary Medicine and the Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. bclee@snu.ac.kr
- 2Institute of Green Bio Science Technology, Seoul National University, Pyeongchang 232-916, Korea.
- 3Emergency Center for Personalized Food-Medicine Therapy System, Advanced Institutes of Convergence Technology, Seoul National University, Suwon 443-270, Korea.
- KMID: 1737624
- DOI: http://doi.org/10.4142/jvs.2014.15.1.167
Abstract
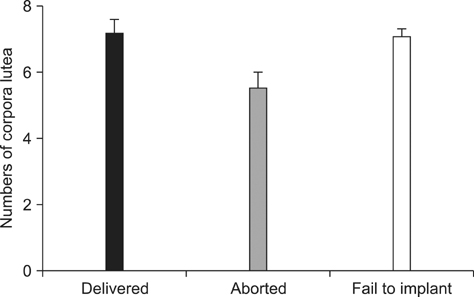
- The level of P4 at the time of embryo transfer (ET) is important. P4 concentrations and numbers of corpora lutea for 126 recipients were evaluated. Nuclear transfer embryos were transferred into 126 surrogates. 11 maintained their pregnancy until full-term delivery, 17 miscarried, and implantation failed in 98 animals. P4 levels in the full-term group were significantly different from those of the pigs that aborted or in which implantation failed (p < 0.05). However, the numbers of corpora lutea were not significantly different. These findings indicate that the concentration of progesterone can be an important factor for successful ET in pigs.
MeSH Terms
Figure
Reference
-
1. Aba MA, Sumar J, Kindahl H, Forsberg M, Edqvist LE. Plasma concentrations of 15-ketodihydro-PGF2α, progesterone, oestrone sulphate, oestradiol-17β and cortisol during late gestation, parturition and the early post partum period in llamas and alpacas. Anim Reprod Sci. 1998; 50:111–121.
Article2. Adair V, Stromer MH, Anderson LL. Progesterone secretion and mitochondrial size of aging porcine corpora lutea. Anat Rec. 1989; 223:252–256.
Article3. Bertoldo MJ, Holyoake PK, Evans G, Grupen CG. Seasonal variation in the ovarian function of sows. Reprod Fertil Dev. 2012; 24:822–834.
Article4. Chen TY, Stott P, Athorn RZ, Bouwman EG, Langendijk P. Undernutrition during early follicle development has irreversible effects on ovulation rate and embryos. Reprod Fertil Dev. 2012; 24:886–892.
Article5. Coster A, Madsen O, Heuven HCM, Dibbits B, Groenen MAM, van Arendonk JAM, Bovenhuis H. The imprinted gene DIO3 is a candidate gene for litter size in pigs. PLoS One. 2012; 7:e31825.
Article6. Dai B, Cao Y, Liu W, Li S, Yang Y, Chen D, Duan E. Dual roles of progesterone in embryo implantation in mouse. Endocrine. 2003; 21:123–132.
Article7. De Rensis F, Saleri R, Tummaruk P, Techakumphu M, Kirkwood RN. Prostaglandin F2α and control of reproduction in female swine: a review. Theriogenology. 2012; 77:1–11.
Article8. Esaki R, Ueda H, Kurome M, Hirakawa K, Tomii R, Yoshioka H, Ushijima H, Kuwayama M, Nagashima H. Cryopreservation of porcine embryos derived from in vitro-matured oocytes. Biol Reprod. 2004; 71:432–437.
Article9. Gao K, Jiang Z, Lin Y, Zheng C, Zhou G, Chen F, Yang L, Wu G. Dietary L-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. 2012; 42:2207–2214.
Article10. Gonzalez-Bulnes A, Torres-Rovira L, Ovilo C, Astiz S, Gomez-Izquierdo E, Gonzalez-Añover P, Pallares P, Perez-Solana ML, Sanchez-Sanchez R. Reproductive, endocrine and metabolic feto-maternal features and placental gene expression in a swine breed with obesity/leptin resistance. Gen Comp Endocrinol. 2012; 176:94–101.
Article11. Joh CH, Kim YJ, Shin ST, Son CH, Lee BC, Lee ES, Rho GJ, Kim IH, Kang TY, Kang HG, Park JI, Cho JK, Yoo IJ, Oh KS, Kim SJ, Lee SL, Jang G. Veterinary obstetrics and theriogenology. Seoul: HongYoungSa;2010. p. 146.12. Koo OJ, Park HJ, Kwon DK, Kang JT, Jang G, Lee BC. Effect of recipient breed on delivery rate of cloned miniature pig. Zygote. 2009; 17:203–207.
Article13. Rátky J, Brüssow KP, Egerszegi I, Torner H, Schneider F, Solti L, Manabe N. Comparison of follicular and oocyte development and reproductive hormone secretion during the ovulatory period in Hungarian native breed, Mangalica, and Landrace gilts. J Reprod Dev. 2005; 51:427–432.
Article14. Robertson HA, King GJ. Plasma concentrations of progesterone, oestrone, oestradiol-17β and of oestrone sulphate in the pig at implantation, during pregnancy and at parturition. J Reprod Fertil. 1974; 40:133–141.
Article15. Ropka-Molik K, Oczkowicz M, Mucha A, Piórkowska K, PiestrzyXMLLink_XYZska-Kajtoch A. Variability of mRNA abundance of leukemia inhibitory factor gene (LIF) in porcine ovary, oviduct and uterus tissues. Mol Biol Rep. 2012; 39:7965–7972.
Article16. Siawrys G, Smolinska N. Direct in vitro effect of LH and steroids on leptin gene expression and leptin secretion by porcine luteal cells during the mid-luteal phase of the estrous cycle. Reprod Biol. 2012; 12:317–323.
Article17. Soede NM, Langendijk P, Kemp B. Reproductive cycles in pigs. Anim Reprod Sci. 2011; 124:251–258.
Article18. Trim CM, Gilroy BA. Cardiopulmonary effects of a xylazine and ketamine combination in pigs. Res Vet Sci. 1985; 38:30–34.
Article19. Wojciechowicz B, Kotwica G, Kolakowska J, Franczak A. The activity and localization of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase and release of androstenedione and progesterone by uterine tissues during early pregnancy and the estrous cycle in pigs. J Reprod Dev. 2013; 59:49–58.
Article20. Yoshizawa M, Watanabe H, Fukui Y. Effects of the presence and the numbers of corpora lutea in non-delivered and delivered pigs on in vitro oocyte maturation and embryonic development. J Reprod Dev. 2009; 55:655–660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Predictive Value of Serum beta Human Chorionic Gonadotropin and Progesterone Measurements for Pregnancy Outcome after In Vitro Fertilizationand Embryo Transfer
- Development of a novel nomogram for predicting ongoing pregnancy after in vitro fertilization and embryo transfer
- The effect of luteal phase progesterone supplementation on natural frozen-thawed embryo transfer cycles
- A high response to controlled ovarian stimulation induces premature luteinization with a negative impact on pregnancy outcomes in a gonadotropin-releasing hormone antagonist cycle
- The Effect of Periovulatory Progesterone Supplementation in In Vitro Fertilization and Embryo Transfer Program